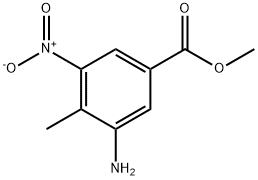
Methyl3-aMino-4-Methyl-5-nitrobenzoate synthesis
- Product Name:Methyl3-aMino-4-Methyl-5-nitrobenzoate
- CAS Number:72922-60-2
- Molecular formula:C9H10N2O4
- Molecular Weight:210.19
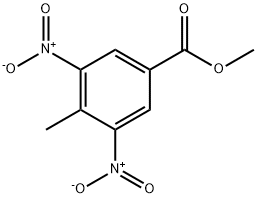
49592-71-4
40 suppliers
inquiry

72922-60-2
29 suppliers
inquiry
Yield:72922-60-2 91.6%
Reaction Conditions:
with iron;acetic acid for 1 h;
Steps:
53.2 Step 2: methyl 3-amino-4-methyl-5-nitrobenzoate (53c)
53b (5 g, 20.8 mmol) was added to AcOH (50 mL). Fe (1.68 g, 3.0 mmol) was added by batches and then the mixture was stirred for 1h. The reaction mixture was then poured into water (200 mL) and extracted with ethyl acetate, the organic layer was dried over magnesium sulfate, filtered, and concentrated in vacuum to give the title 53c (4 g, 91.6%). LC-MS (ESI): m/z =211.3 [M+H] +
References:
WO2020/185755,2020,A1 Location in patent:Paragraph 00613
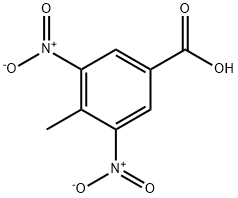
16533-71-4
220 suppliers
$9.00/10g

72922-60-2
29 suppliers
inquiry