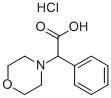
MORPHOLIN-4-YL-PHENYL-ACETIC ACID HYDROCHLORIDE synthesis
- Product Name:MORPHOLIN-4-YL-PHENYL-ACETIC ACID HYDROCHLORIDE
- CAS Number:91641-50-8
- Molecular formula:C12H16ClNO3
- Molecular Weight:257.7133
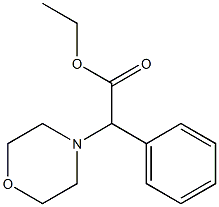
22083-23-4

91641-50-8
Preparation of 2-morpholino-2-phenylacetic acid hydrochloride (I39): ethyl 2-morpholino-2-phenylacetate (0.34 g, 1.36 mmol) was dissolved in dioxane (11.4 mL) and 37% hydrochloric acid (1.1 mL, 13.6 mmol) was added slowly and dropwise. The reaction mixture was stirred under reflux conditions overnight. 37% hydrochloric acid (1.1 mL, 13.6 mmol) was added again and the reaction was continued at reflux for 24 hours. Upon completion of the reaction, the solvent was removed by evaporation and the residue was ground with acetonitrile (10 mL) to afford 2-morpholino-2-phenylacetic acid hydrochloride as a white solid (0.22 g, 63.7% yield).1H NMR (300 MHz, DMSO-d6) δ 11.79 (s, 1H), 7.56-7.72 (m, 2H), 7.37-7.56 (m, 3H) , 5.25 (s, 1H), 3.60-4.10 (m, 4H), 3.27 (br.s, 2H), 2.96 (br.s, 2H).

22083-23-4
0 suppliers
inquiry

91641-50-8
17 suppliers
$137.75/1g
Yield:91641-50-8 63.7%
Reaction Conditions:
with hydrogenchloride;water in 1,4-dioxane;Reflux;
Steps:
12 Preparation of 2-morpholino-2-phenylacetic acid hydrochloride (139).
Preparation of 2-morpholino-2-phenylacetic acid hydrochloride (139). Ethyl 2-moφholino-2-phenylacetate (0.34 g, 1.36 mmol) was dissolved in dioxane (1 1.4 ml) and 37% HCl (1.1 ml, 13.6 mmol) was added dropwise. The mixture was stirred at reflux overnight. 37% HCl (1.1 ml, 13.6 mmol) was added and the reaction was refluxed for additional 24 h. The solvent was evaporated and the residue was triturated with acetonitrile (10 ml) to obtain 2-moφholino-2-phenylacetic acid hydrochloride (0.22 g, 63.7 % yield) as a white solid. 1H NMR (300 MHz, DMSO-d6) ppm 1 1.79 (s, 1 H), 7.56 - 7.72 (m, 2 H), 7.37 - 7.56 (m, 3 H), 5.25 (s, 1 H), 3.60 - 4.10 (m, 4 H), 3.27 (br. s., 2 H), 2.96 (br. s., 2 H).
References:
WO2013/98145,2013,A1 Location in patent:Page/Page column 50

100609-91-4
0 suppliers
inquiry

91641-50-8
17 suppliers
$137.75/1g
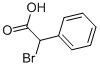
4870-65-9
197 suppliers
$8.00/1g

91641-50-8
17 suppliers
$137.75/1g
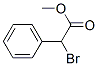
37167-62-7
19 suppliers
inquiry

91641-50-8
17 suppliers
$137.75/1g
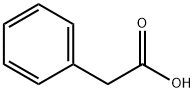
103-82-2
0 suppliers
$27.60/100g

91641-50-8
17 suppliers
$137.75/1g