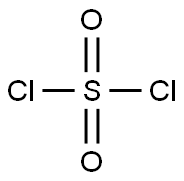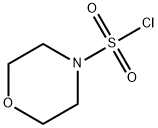
MORPHOLINE-4-SULFONYL CHLORIDE synthesis
- Product Name:MORPHOLINE-4-SULFONYL CHLORIDE
- CAS Number:1828-66-6
- Molecular formula:C4H8ClNO3S
- Molecular Weight:185.63

110-91-8

1828-66-6
1. 10.0 mL of dichloromethane (CH2Cl2) was added to a 50 mL round bottom flask and cooled in an ice bath. 2. 0.300 mL of sulfuryl chloride (SO2Cl2) was slowly added to the cooled dichloromethane. 3. 213 mg of morpholine was dissolved in 3.0 mL of dichloromethane and slowly added to the above reaction mixture, kept cool in an ice bath. 4. 520 mg of trimethylamine was added and the reaction mixture was stirred at room temperature for about 2 hours. 5. Upon completion of the reaction, the product was dissolved with 20.0 mL of chloroform and subsequently washed with 20.0 mL of ice water. 6. The chloroform phase was separated, dried over magnesium sulfate (MgSO4) and filtered. 7. The filtrate was distilled under pressure to give 160 mg of morpholine-4-sulfonyl chloride in about 23.3% yield. 8. 70.0 mg of (R)-N-methyl-N-(pyrrolidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine was added to a 5 mL round-bottomed flask and dissolved in 1.00 mL of dichloromethane. 9. 0.0390 mL morpholine-4-sulfonyl chloride was added, followed by 0.0590 mL N,N-diisopropylethylamine and stirred overnight at room temperature. 10. The reaction mixture was concentrated under pressure and the residue was purified by fast column chromatography (MeOH:CH2Cl2 = 2:98). 11. The purified grades were collected, concentrated under reduced pressure and further dried under vacuum to give 47.0 mg (R)-N-methyl-N-(1-(morpholinosulfonyl)pyrrolidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine in about 40.2% yield. 12. The product was analyzed by 1H NMR. 12. The product was structurally confirmed by 1H NMR and LRMS (ESI).

110-91-8
710 suppliers
$9.00/1g

1828-66-6
90 suppliers
$8.00/1g
Yield:1828-66-6 100%
Reaction Conditions:
with sulfuryl dichloride in acetonitrile for 24 h;Heating / reflux;
Steps:
126
Morpholine (0.436 mL, 5 mmol) was added slowly and with caution to a mixture of sulfuryl chloride (1.205 mL, 15 mmol) in 5 mL acetonitrile. Heated to reflux and stirred for 24 hours. After starting material consumed, solution concentrated to oil, azeotroped with toluene (2x), concentrated to give crude product 126 stored as a 2M solution in dichloromethane (0.999 g, 5 mmol, 100%. ) ¹H NMR (CD3SOCD3) No. 3.80 (br s, 4H), 3.28 (br s, 4H.) MS: 186 (M+1)
References:
GILEAD SCIENCES, INC.;CAI, Zhenhong, R.;CHEN, Xiaowu;FARDIS, Maria;JABRI, Salman, Y.;JIN, Haolun;KIM, Choung, U.;METOBO, Sanuel, E.;MISH, Michael, R.;PASTOR, Richard, M. WO2005/117904, 2005, A2 Location in patent:Page/Page column 463-464
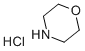
10024-89-2
129 suppliers
$5.00/5g

1828-66-6
90 suppliers
$8.00/1g
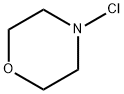
23328-69-0
20 suppliers
$148.50/1g

1828-66-6
90 suppliers
$8.00/1g
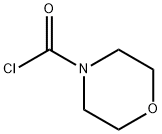
15159-40-7
140 suppliers
$13.43/250mgs:

1828-66-6
90 suppliers
$8.00/1g