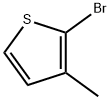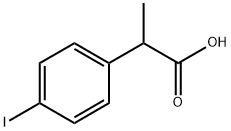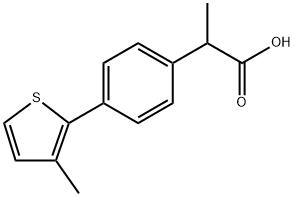
MTPPA synthesis
- Product Name:MTPPA
- CAS Number:70991-61-6
- Molecular formula:C14H14O2S
- Molecular Weight:246.32
Yield:70991-61-6 95%
Reaction Conditions:
with hydrogenchloride;magnesium;Zinc chloride;palladium(II) chloride in tetrahydrofuran;sodium hydroxide;cyclohexane;water;toluene;
Steps:
12.3 (3)
(3) Synthesis of 2-[4-(3-methyl-2-thienyl)phenyl]propionic acid In 84 ml of anhydrous tetrahydrofuran, 27.8 g (0.157 mole) of 2-bromo-3-methyl-thiophene was reacted with 4.2 g (0.173 mole) of magnesium turnings to form a Grignard reagent. To a solution of 34 g (0.123 mole) of 2-(4-iodophenyl)propionic acid in 125 ml of 1 N aqueous sodium hydroxide solution was added 9.2 g (0.0676 mole) of anhydrous zinc chloride to form zinc salt of said acid. The zinc salt was extracted with 100 ml of toluene and the extract solution was azeotropically dehydrated and then freed from the solvent by distillation under reduced pressure. To the residue was added 100 ml of tetrahydrofuran, followed by adding 0.035 g of palladium chloride. To the resulting mixture heated to 60° to 65° C. was added dropwise with stirring the Grignard reagent prepared above, to allow the reaction to proceed. After completion of the addition, the mixture was heated under reflux for 2 hours, then cooled, and freed from the solvent by distillation under reduced pressure. To the residue were added 100 ml of water, 12.5 ml of concentrated hydrochloric acid and 100 ml of toluene, with stirring. The organic layer was separated, washed several times with water, and mixed with 125 ml of 1 N aqueous sodium hydroxide solution to extract the alkali-soluble reaction products. The aqueous layer was separated, and 100 ml of cyclohexane was added thereto, after which the mixture was adjusted to pH 4 with 6 N hydrochloric acid while stirring. The organic layer was separated, washed with water, and dried over anhydrous magnesium sulfate. The magnesium sulfate was removed by filtration and the filtrate was continually stirred at 10° to 20° C. to precipitate crystals. The crystals were collected by filtration to obtain 26 g (95% yield) of 2-[4-(3-methyl-2-thienyl)phenyl]propionic acid. Melting point: 98°-99° C. (recrystallized from a cyclohexane-ethanol mixture). I.R. (KBr): νC=O 1700 cm-1. Elementary analysis for C14 H14 O2 S:
References:
US4230719,1980,A
70991-60-5
1 suppliers
inquiry

70991-61-6
5 suppliers
inquiry
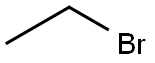
74-96-4
430 suppliers
$9.00/5g
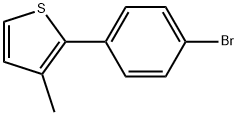
70991-68-3
1 suppliers
inquiry
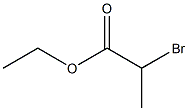
41978-69-2
4 suppliers
inquiry

70991-61-6
5 suppliers
inquiry