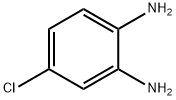
N*1*-BENZYL-4-CHLORO-BENZENE-1,2-DIAMINE synthesis
- Product Name:N*1*-BENZYL-4-CHLORO-BENZENE-1,2-DIAMINE
- CAS Number:39235-92-2
- Molecular formula:C13H13ClN2
- Molecular Weight:232.71
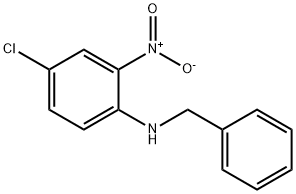
10066-18-9
10 suppliers
inquiry

39235-92-2
12 suppliers
inquiry
Yield:39235-92-2 98%
Reaction Conditions:
with iron(0);ammonia hydrochloride in ethanol;lithium hydroxide monohydrate at 80;Bechamp Arsonilation;
Steps:
General procedure for the synthesis of diamines 49a-g
Example for 49a: N-benzylnitroaniline 48a (1.6 g, 6.10 mmol, 1 equiv) was dissolved in hot EtOH (50mL). Iron powder (2.0 g, 36.6 mmol, 6 equiv) was added followed by a solution of ammonium chloride(1.9 g, 36.6 mmol, 6 equiv) in water (50 mL). The mixture was heated at 80 °C until the reaction wascompleted (1-3 h), monitoring by TLC or HPLC. After cooling, the reaction was quenched with asaturated solution of sodium bicarbonate (50 mL) and the mixture was partially evaporated. The residuewas treated with CH2Cl2 (300 mL) and washed with a saturated solution of sodium bicarbonate (3 x 100mL) and brine (3 x 100 mL). After drying over MgSO4, the organic phase was concentrated to dryness,affording the corresponding diamine 49a.N1-benzyl-4-chlorobenzene-1,2-diamine (49a). (1.4 g, 98% yield). 1H NMR (500 MHz, CDCl3) d 7.41- 7.27 (m, 5H), 6.76 - 6.69 (m, 2H), 6.55 (d, J = 8.3 Hz, 1H), 4.28 (s, 2H), 3.42 (bs, 2H). 13C NMR(126 MHz, CDCl3) d 138.97, 136.05, 135.61, 128.69, 127.75, 127.43, 123.64, 120.03, 116.15, 112.94,48.71. HRMS (ESI-QTOF) m/z: [M + H]+ calcd for C13H13ClN2, 233.0840; found, 233.0869.
References:
Deodato, Davide;Asad, Nadeem;Dore, Timothy M. [Bioorganic and Medicinal Chemistry Letters,2022,vol. 72,art. no. 128867] Location in patent:supporting information
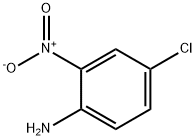
89-63-4
31 suppliers
$13.00/50g

39235-92-2
12 suppliers
inquiry

100-39-0
430 suppliers
$10.00/10g

39235-92-2
12 suppliers
inquiry