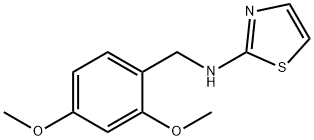
N-(2,4-diMethoxybenzyl)thiazol-2-aMine synthesis
- Product Name:N-(2,4-diMethoxybenzyl)thiazol-2-aMine
- CAS Number:853994-53-3
- Molecular formula:C12H14N2O2S
- Molecular Weight:250.32
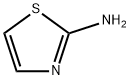
96-50-4
555 suppliers
$5.00/5g
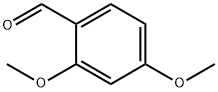
613-45-6
499 suppliers
$10.00/5g

853994-53-3
28 suppliers
$150.96/250mg
Yield:853994-53-3 65%
Reaction Conditions:
Stage #1: 2-thiazolylamine;2,4-Dimethoxybenzaldehyde in toluene at 105; for 0.5 h;
Stage #2: with sodium tris(acetoxy)borohydride in toluene at 35; for 0.5 h;
Steps:
8-1 8-1)
N-(2,4-Dimethoxybenzyl)-1,3-thiazol-2-amine (Compound L)
A solution of 2-aminothiazole (5.0 g, 49.9 mmol) and 2,4-dimethoxybenzaldehyde (9.96 g, 59.9 mmol) in toluene (60 mL) was stirred at approximately 105° C. for 30 minutes.
The reaction solution was cooled to approximately 35° C. Then, sodium triacetoxyborohydride (14.8 g, 69.9 mmol) was added thereto, and the mixture was stirred at approximately 35° C. for 30 minutes.
To the reaction solution, ethyl acetate (100 mL) and water (50 mL) were added, followed by extraction.
Then, the organic layer was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (hexane/ethyl acetate: 1/1).
2-Propanol (20 mL) was added to the obtained solid, and the mixture was stirred at room temperature for 15 minutes.
Water (50 mL) was added thereto, and the mixture was stirred for 30 minutes.
Then, the resulting solid was collected by filtration and dried under reduced pressure to obtain the title compound (8.13 g, 65%) as a white solid.
1H-NMR (500 MHz, CDCl3) δ ppm: 3.79 (3H, s), 3.82 (3H, s), 4.38 (2H, s), 5.69 (1H, br s), 6.43 (1H, dd, J=2.0, 8.0 Hz), 6.46-6.47 (2H, m), 7.09 (1H, d, J=3.5 Hz), 7.22 (1H, d, J=8.0 Hz).
References:
US2018/251448,2018,A1 Location in patent:Paragraph 0341; 0342

613-45-6
499 suppliers
$10.00/5g

853994-53-3
28 suppliers
$150.96/250mg