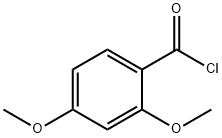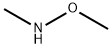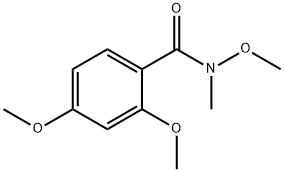
N,2,4-trimethoxy-N-methylbenzamide synthesis
- Product Name:N,2,4-trimethoxy-N-methylbenzamide
- CAS Number:206051-18-5
- Molecular formula:C11H15NO4
- Molecular Weight:225.24
Yield:206051-18-5 93%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in N,N-dimethyl-formamide at 20; for 3 h;
Steps:
4.2.4. N,2-Dimethoxy-N-methylbenzamide (9a)
General procedure: To a solution of 3-cyanobenzoic acid 8a (3.0 g, 19.7 mmol) in DMF was added N,O-dimethylhydroxylamine hydrochloride (2.0 g, 20.7 mmol), Et3N (2.88 mL, d = 0.73, 20.7 mmol) and EDC·HCl (4.0 g, 20.7 mmol). After the mixture was stirred for 3 h at room temperature, the solvent was removed in vacuo and the residue was dissolved in EtOAc, washed with 10% citric acid, 10% NaHCO3 and saturated NaCl, and dried over Na2SO4. Then, the solvent was removed to give a colorless oil of compound 9a (3.0 g, 79%).
References:
Hayashi, Yoshio;Yamazaki, Yuri;Sumikura, Makiko;Masuda, Yurika;Hayashi, Yoshiki;Yasui, Hiroyuki;Kiso, Yoshiaki;Chinen, Takumi;Usui, Takeo;Yakushiji, Fumika;Potts, Barbara;Neuteboom, Saskia;Palladino, Michael;Lloyd, George Kenneth [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 14,p. 4279 - 4289] Location in patent:experimental part
91-52-1
229 suppliers
$10.60/1gm:
30289-28-2
39 suppliers
$45.00/25mg

206051-18-5
5 suppliers
inquiry

91-52-1
229 suppliers
$10.60/1gm:

206051-18-5
5 suppliers
inquiry