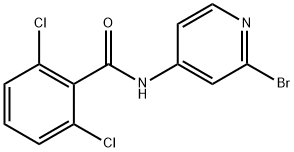
N-(2-BroMopyridin-4-yl)-2,6-dichlorobenzaMide synthesis
- Product Name:N-(2-BroMopyridin-4-yl)-2,6-dichlorobenzaMide
- CAS Number:1258298-00-8
- Molecular formula:C12H7BrCl2N2O
- Molecular Weight:346.01
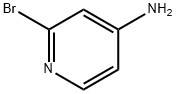
7598-35-8
365 suppliers
$6.00/250mg
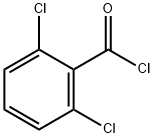
4659-45-4
287 suppliers
$12.00/5g

1258298-00-8
16 suppliers
$319.00/250mg
Yield:1258298-00-8 74%
Reaction Conditions:
Stage #1: 2-bromo-4-aminopyridinewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 0.333333 h;
Stage #2: 2,6-Dichlorobenzoyl chloride in N,N-dimethyl-formamide;mineral oil at 0; for 4 h;
Steps:
2.1
A solution of 2-bromopyridin-4-amine (0.74 g, 4.3 mmol) in DMF (5 mL) was added to a cooled (0° C.) mixture of NaH (0.31 g, 7.82 mmol, 60% in mineral oil) in DMF (15 mL). The resulting mixture was stirred for 20 minutes at 0° C. and then a solution of 2,6-dichlorobenzoyl chloride (0.82 g, 3.91 mmol) in DMF (5 mL) was added dropwise. The reaction was stirred at 0° C. for 4 hours and then poured onto ice-water (20 mL). The precipitate was collected and filtrate was extracted by EtOAc (2×50 mL). The combined organic extracts were dried over Na2SO4 and concentrated under reduced pressure. The resulting solid was combined with the precipitate to afford N-(2-bromopyridin-4-yl)-2,6-dichlorobenzamide (1.0 g, Yield: 74%). 1HNMR (DMSO-d6, 400 MHz): δ 11.41 (s, 1H), 8.33 (d, J=5.6 Hz, 1H), 7.99 (d, J=1.6 Hz, 1H), 7.64-7.54 (m, 4H). LCMS (ESI) m/z: 345.0 [M+H+].
References:
US2010/317643,2010,A1 Location in patent:Page/Page column 49
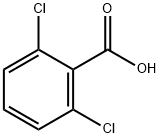
50-30-6
363 suppliers
$5.00/5g

1258298-00-8
16 suppliers
$319.00/250mg