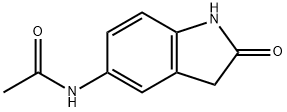
N-(2-Oxo-2,3-dihydro-1H-indol-5-yl)-acetaMide synthesis
- Product Name:N-(2-Oxo-2,3-dihydro-1H-indol-5-yl)-acetaMide
- CAS Number:114741-27-4
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2
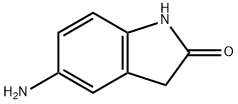
20876-36-2
181 suppliers
$16.00/250mg

75-36-5
592 suppliers
$17.92/100G

114741-27-4
8 suppliers
inquiry
Yield:114741-27-4 89%
Reaction Conditions:
with triethylamine in tetrahydrofuran at -30 - 20;
Steps:
30.3
5-Amino-1,3-dihydro-indol-2-one 30c (3.5 g, 23.6 mmol) was dissolved in 20 ml of tetrahydrofuran under stirring, and added with triethylamine (3.6 ml, 26 mmol) to the solution at room temperature. Upon completion of the addition, the mixture was cooled down to -30° C. in a dry ice-acetone bath, and added slowly with acetyl chloride (1.8 ml, 24.8 mmol) while maintaining the temperature below -20° C. Upon completion of the addition, the dry ice-acetone bath was removed, and the reaction mixture was allowed to warm up to room temperature and stirred for 20 minutes.After thin lay chromatography showed the disappearance of starting materials, the reaction mixture was added with ethyl acetate (20 ml), gray solids were formed and filtered. The filter cake was washed with water (70 ml×3) to obtain 2.5 g of solids. The filtrate was extracted with ethyl acetate (200 ml×3). The combined organic extracts were concentrated under reduced pressure, combined with the above solids to obtain the title compound N-(2-oxo-2,3-dihydro-1H-indol-5-yl)-acetamide 30d (4.0 g, yield 89%) as a gray solid.MS m/z (ESI): 191.2 [M+1]
References:
US2010/75952,2010,A1 Location in patent:Page/Page column 64

20876-36-2
181 suppliers
$16.00/250mg

108-24-7
0 suppliers
$14.00/250ML

114741-27-4
8 suppliers
inquiry

20876-36-2
181 suppliers
$16.00/250mg

114741-27-4
8 suppliers
inquiry
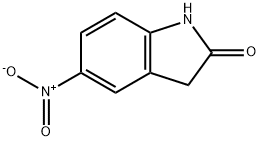
20870-79-5
186 suppliers
$7.00/250mg

108-24-7
0 suppliers
$14.00/250ML

114741-27-4
8 suppliers
inquiry

20876-36-2
181 suppliers
$16.00/250mg

64-19-7
1629 suppliers
$10.00/25ML

114741-27-4
8 suppliers
inquiry