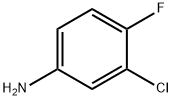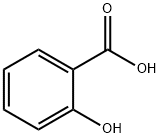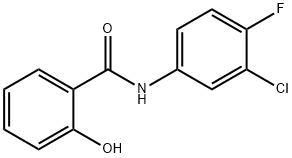
N-(3-chloro-4-fluorophenyl)-2-hydroxybenzamide synthesis
- Product Name:N-(3-chloro-4-fluorophenyl)-2-hydroxybenzamide
- CAS Number:341018-39-1
- Molecular formula:C13H9ClFNO2
- Molecular Weight:265.67
Yield:341018-39-1 96%
Reaction Conditions:
Stage #1: 3-chloro-4-fluorophenylamine;salicylic acid in 5,5-dimethyl-1,3-cyclohexadiene at 120; for 0.5 h;
Stage #2: with phosphorus trichloride in 5,5-dimethyl-1,3-cyclohexadiene at 120; for 3 h;
Steps:
General procedure for the synthesis of compounds 3-25
General procedure: To a mixture of 5-chlorosalicylic acid (1a-d, 1 mmol) andaniline derivative (2a-k, 1 mmol) in a round bottom flasadded 10 mL xylenes mixture (mixture of o-, m-, and pxylenes)and heated to 120 °C for 30 min.PCl3(0.4 mmol)was added dropwise for 5 min, then the reaction contentsstirred at 120 °C for 3-4 h. The reaction progress wasmonitored by TLC carried out on Merck silica-gel plates(0.25 mm thick, 60F254) in ethylacetate-hexanes (1:3) andvisualized by UV (254 nm) light. After complete consumptionof starting materials (observed by TLC), thecontents were brought to 70 °C, and then quenched withwater. The resulting white precipitate was filtered and washed with water (20 mL, 70 °C), obtained desired salicylanilidewith >96% purity. Further washing with 10%ethanol in water (20 mL) removed trace amount (3-4%) ofunreacted aniline derivative. The wet white solid was driedunder reduced pressure for 3-4 h, resulted desired compounds(3-25) with >98% purity.
References:
Lal, Jhajan;Kaul, Grace;Akhir, Abdul;Ansari, Shabina B.;Chopra, Sidharth;Reddy, Damodara N. [Medicinal Chemistry Research,2021,vol. 30,# 12,p. 2301 - 2315]