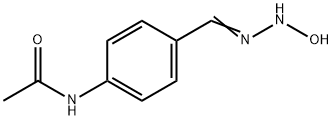
N-[4-(N-HYDROXYCARBAMIMIDOYL)-PHENYL]-ACETAMIDE synthesis
- Product Name:N-[4-(N-HYDROXYCARBAMIMIDOYL)-PHENYL]-ACETAMIDE
- CAS Number:6577-60-2
- Molecular formula:C9H11N3O2
- Molecular Weight:193.2
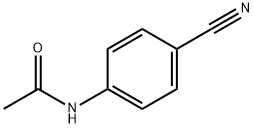
35704-19-9
120 suppliers
$12.00/5g
![N-[4-(N-HYDROXYCARBAMIMIDOYL)-PHENYL]-ACETAMIDE](/CAS/GIF/6577-60-2.gif)
6577-60-2
4 suppliers
inquiry
Yield:6577-60-2 94.07%
Reaction Conditions:
with hydroxylamine hydrochloride;triethylamine in ethanol at 100; for 71 h;
Steps:
4.2.4 Synthesis of N-(4-(N'-hydroxycarbamimidoyl)phenyl)acetamide (IIIb)
Hydroxylammonium chloride (14.9mmol; 1.04g) was added to anhydrous ethanol (100mL) under stirring condition, followed by triethylamine (14.9mmol; 2.1mL). After stirring until everything is dissolved (about 15min) compound IIb (12.5mmol; 2g) was added and heated at boiling point (100 °C). As the reaction proceeds slowly, 0.21g of hydroxylammonium chloride and 420μL of triethylamine are added after 23h. After 48h, the reaction was completed, and the reaction mixture evaporated. A mixture of ice and water (75mL) was then added and the precipitate formed was filtered off by suction and washed with a little ice water and dried. N-(4-(N'-hydroxycarbamimidoyl)phenyl)acetamide (IIIb). Yield: 94.07%; Yellowish powder; Rf: 0.13 (MF: chloroform/methanol=9/1); 1H NMR (400MHz, DMSO-d6): δ[ppm] 2.07 (s, 3H, CH3CO); 5.74 (s, 2H, NH2); 7.26-7.58 (m, 4H, 4H Ar); 9.52 (s, 1H, OH); 10.03 (s, 1H, NH); IR (cm-1): 3241, 1638, 1594, 1540, 1406, 1369, 1336, 1307, 1276, 1185, 958, 837, 814, 732, 685, 611, 571, 514; MS (ESI+): 193.95 [M+H]+ (calculated 193.203); Melting point: 150-152°C.
References:
Dolenc, Marija Sollner;Loboda, Kaja Bergant;Perdih, Andrej;Valjavec, Katja;Wolber, Gerhard;?tampar, Martina;?egura, Bojana;Filipi?, Metka [Bioorganic Chemistry,2020,vol. 99]
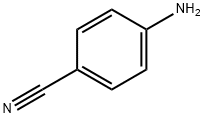
873-74-5
651 suppliers
$9.00/1g
![N-[4-(N-HYDROXYCARBAMIMIDOYL)-PHENYL]-ACETAMIDE](/CAS/GIF/6577-60-2.gif)
6577-60-2
4 suppliers
inquiry