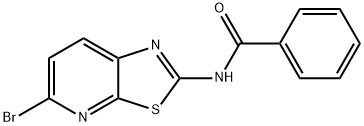
N-(5-bromothiazolo[5,4-b]pyridin-2-yl)benzamide synthesis
- Product Name:N-(5-bromothiazolo[5,4-b]pyridin-2-yl)benzamide
- CAS Number:1107694-72-3
- Molecular formula:C13H8BrN3OS
- Molecular Weight:334.19
![Benzamide, N-[[(6-bromo-2-chloro-3-pyridinyl)amino]thioxomethyl]-](/CAS/20200515/GIF/1256956-09-8.gif)
1256956-09-8
0 suppliers
inquiry
![N-(5-bromothiazolo[5,4-b]pyridin-2-yl)benzamide](/CAS/20180601/GIF/1107694-72-3.gif)
1107694-72-3
9 suppliers
inquiry
Yield:1107694-72-3 76%
Reaction Conditions:
with sodium ethanolate in 1-methyl-pyrrolidin-2-one at 120; for 8 h;
Steps:
13.2
A solution of N-(6-bromo-2-chloropyridin-3-ylcarbamothioyl)benzamide (1.5 g, 4.0 mmol) and sodium ethoxide (0.54 g, 8.0 mmol) in l-methyl-2-pyrrolidinone (10 mL) was heated to 120 °C for 8 hours. After cooling the reaction mixture to room temperature the mixture was poured into water. The resulting solid was collected by filtration, then washed sequentially with water and diethyl ether. The filter cake was dried to give N-(5-bromothiazolo[5,4-b]pyridin-2-yl)benzamide (1.02 g, 76%). 1H NMR (400 MHz, de-DMSO): 13.2 (br s, 1H), 8.16-8.10 (m, 3H), 7.72 (d, 1H), 7.70 (t, 1H), 7.59 (t, 2H). MS (EI) for C13H8BrN3OS: 336 (MH+).
References:
WO2010/135524,2010,A1 Location in patent:Page/Page column 208