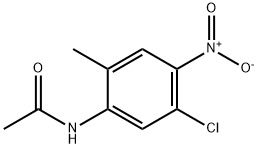
N-(5-Chloro-2-methyl-4-nitrophenyl)-acetamide synthesis
- Product Name:N-(5-Chloro-2-methyl-4-nitrophenyl)-acetamide
- CAS Number:13852-50-1
- Molecular formula:C9H9ClN2O3
- Molecular Weight:228.63
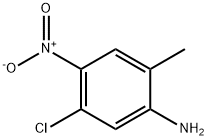
13852-51-2
159 suppliers
$8.00/250mg

75-36-5
589 suppliers
$17.92/100G

13852-50-1
14 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 5-chloro-2-methyl-4-nitroanilinewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2: acetyl chloride in N,N-dimethyl-formamide at 0; for 0.166667 h;
Steps:
b b) Synthesis of tert-butyl (2,5-dimethyl-4-nitrophenyl)carbamate:
To a stirred solution of 5-chloro-2-methyl-4-nitroaniline (12 g, 64.3 mmol) in N, N-d met hy I for ma m i de (80 mL), sodium hydride (3.09 g, 129 mmol) was added and the reaction mixture was stirred at 0 °C for 30 min, followed by addition of a solution of acetyl chloride (6.06 mL, 77 mmol)) in N,N- dimethylformamide (80 mL) at 0 °C within 10 min. After completion of the reaction, the reaction mixture was warmed to 25 °C, diluted with ethyl acetate and filtered. The combined filtrate was diluted with water (100 mL) and extracted three times with ethyl acetate (100 mL). The combined organic layers were washed two times with cold water (100 mL) followed by brine (50 mL), dried over anhydrous sodium sulphate, filtered and concentarted under reduced pressure to obtain N-(5-chloro-2-methyl-4- nitrophenyl)acetamide (8 g, 35.0 mmol, 54 % yield) LCMS (M+l) 230.1, which used directly for the next step.
References:
WO2020/35825,2020,A1 Location in patent:Page/Page column 67-68
5900-55-0
63 suppliers
inquiry

13852-50-1
14 suppliers
inquiry

13852-51-2
159 suppliers
$8.00/250mg

108-24-7
0 suppliers
$14.00/250ML

75-36-5
589 suppliers
$17.92/100G

13852-50-1
14 suppliers
inquiry
7664-93-9
0 suppliers
$23.30/1090731000

5900-55-0
63 suppliers
inquiry
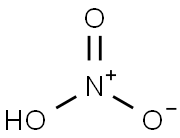
7697-37-2
0 suppliers
$13.00/25g

64-19-7
1627 suppliers
$10.00/25ML

13852-50-1
14 suppliers
inquiry

13852-52-3
4 suppliers
inquiry