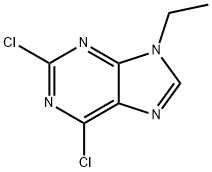
9H-?Purine, 2,?6-?dichloro-?9-?ethyl- synthesis
- Product Name:9H-?Purine, 2,?6-?dichloro-?9-?ethyl-
- CAS Number:190655-14-2
- Molecular formula:C7H6Cl2N4
- Molecular Weight:217.06

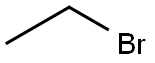
75-03-6
367 suppliers
$11.00/5g
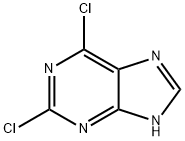
5451-40-1
502 suppliers
$10.00/1g

190655-14-2
23 suppliers
inquiry
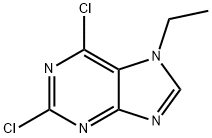
190654-80-9
15 suppliers
inquiry
Yield:190655-14-2 82% ,190654-80-9 87%
Reaction Conditions:
Stage #1: 2,6 dichloropurinewith sodium carbonate in acetone; for 0.333333 h;Heating;Reflux;
Stage #2: ethyl iodide in acetone; for 5 h;Heating;Reflux;
Steps:
6.1
Example 66-(3-chlorophenylamino)-9-ethyl-9H-purine-2-carbonitrile (32)General procedure for the formation of 2,6-dichloro-9-alkyl-9H-purines (Step 1): 2,6-dichloro-9-ethyl-9H-purine - To a solution of 2,6-dichloro-9H-purine (2 g, 10.58 mmol) in acetone (45 ml) was added sodium carbonate (2.25 g, 21.16 mmol). The reaction vessel was equipped with a reflux condenser, and the mixture was heated under reflux conditions for 20 minutes. After that time, iodoethane (0.855 ml, 10.58 mmol) was added in one portion, and the reaction mixture was allowed to stir for 5 hrs. Upon completion, the reaction mixture was concentrated under reduced pressure and directly purified on silica column. Gradient elution with ethyl acetate (2→40%) in hexanes provided regioisomers 2,6-dichloro-9-ethyl-9H-purine and 2,6-dichloro-7-ethyl-7H-purine as pale yellow solids: yield (1.5 g, 6.91 mmol, 82 %; 0.4 g, 1.843 mmol, 87 %, respectively).
References:
WO2010/59418,2010,A1 Location in patent:Page/Page column 61-62

75-03-6
367 suppliers
$11.00/5g

5451-40-1
502 suppliers
$10.00/1g

190655-14-2
23 suppliers
inquiry

190654-80-9
15 suppliers
inquiry