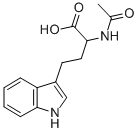
N-Acetyl-D,L-homotryptophan synthesis
- Product Name:N-Acetyl-D,L-homotryptophan
- CAS Number:205813-00-9
- Molecular formula:C14H16N2O3
- Molecular Weight:260.29
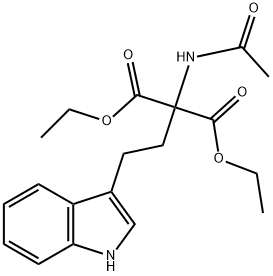
351421-21-1
17 suppliers
$218.50/25mg

205813-00-9
20 suppliers
$248.00/10mg
Yield:205813-00-9 56%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;water; for 15 h;Reflux;
Steps:
2-Acetamido-4-(1H-indol-3-yl)butanoic acid (5a)
In a round bottom flask, NaOH (0.225 g, 1.2 eq.) was dissolved in aqueous tetrahydrofuran (2:1, THF:H2O, 45 ml) solution. Then compound 4a (1.69 g, 1 eq.) was added in and the solution was allowed to reflux for 15 h. The solvent was then removed under reduced pressure and the residue was taken up in water and ethyl acetate. The aqueous layer was acidified to pH 2 using 6 M HCl and extracted with ethyl acetate. The organic layer was washed with water and dried over MgSO4. Removal of solvent under reduced pressure give crude 5a as a yellow solid which can be further purified by recrystallization in water to give pure 5a as an off-white needle-shaped solid. Yield: 0.684 g (56%). 1H NMR (DMSO) δ (ppm): 1.87 (s, 3H), 1.98-2.01 (m, 2H), 2.63-2.74 (m, 2H), 4.15-4.20 (m, 1H), 6.93-6.97 (t, 1H), 7.02-7.06 (t, 1H), 7.07 (s, 1H), 7.30-7.32 (d, 1H), 7.48-7.49 (d, 1H), 8.22-8.24 (1H), 12.51 (broad, 1H).
References:
Do, Quang T.;Nguyen, Giang T.;Celis, Victor;Phillips, Robert S. [Archives of Biochemistry and Biophysics,2014,vol. 560,p. 20 - 26]
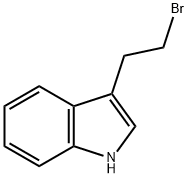
3389-21-7
128 suppliers
$19.00/250mg

205813-00-9
20 suppliers
$248.00/10mg
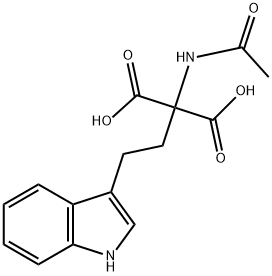
408537-42-8
14 suppliers
$150.00/25mg

205813-00-9
20 suppliers
$248.00/10mg
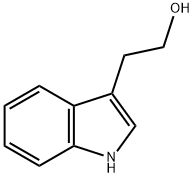
526-55-6
324 suppliers
$5.00/50mg

205813-00-9
20 suppliers
$248.00/10mg