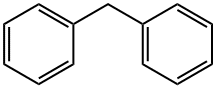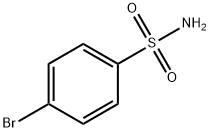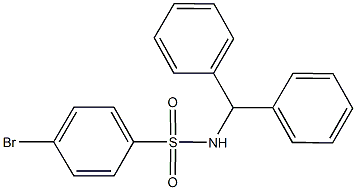
N-benzhydryl-4-bromobenzenesulfonamide synthesis
- Product Name:N-benzhydryl-4-bromobenzenesulfonamide
- CAS Number:853-82-7
- Molecular formula:C19H16BrNO2S
- Molecular Weight:402.3048
Yield:853-82-7 86%
Reaction Conditions:
with 2,3-dicyano-5,6-dichloro-p-benzoquinone in 1,2-dichloro-ethane at 100; for 24 h;Schlenk technique;Molecular sieve;Inert atmosphere;
Steps:
2.2 Procedure for the transition metal-free oxidative coupling of diphenylmethanes with TsNH2
General procedure: A 20 mL Schlenk tube equipped with a stir-bar was charged with substituted diphenylmethanes (0.5 mmol), TsNH2 (1.0 mmol), DDQ (0.6 mmol) and 200.0 mg 3 ? molecular sieve. The reaction tube was purged with nitrogen. 4 mL 1,2-dichloroethane (DCE) was then added to the reaction tube via a syringe. The Schlenk tube was placed in an oil-bath and heated to 100°C for 24 h. The reaction mixture was then cooled to room temperature and filtered with ethyl acetate (2×10 mL) to remove the solid residue. The combined solution was concentrated by the rotary evaporator, and then purified by column chromatography on silica gel using petroleum ether/ethyl acetate as eluent to give the desired product.
References:
Liu, Jie;Zhang, Heng;Yi, Hong;Liu, Chao;Lei, Aiwen [Science China Chemistry,2015,vol. 58,# 8,p. 1323 - 1328]