
N-benzyl-2-chloroquinazolin-4-amine synthesis
- Product Name:N-benzyl-2-chloroquinazolin-4-amine
- CAS Number:157864-32-9
- Molecular formula:C15H12ClN3
- Molecular Weight:269.73
Yield:157864-32-9 97%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in ethanol at 21;
Steps:
General Method D
General procedure: A stirring solution of amine or amine salt (4.21mmol) and DIPEA (1.0mL, 5.7mmol) in EtOH (10mL) was treated with the appropriate 2,4-dichloroquinazoline, 2,4-dichloropyrimidine or related compound (794mg, 3.99mmol) at 21°C until adjudged complete by TLC or LCMS. The reaction mixture was diluted using H2O (8mL) and saturated KH2PO4 (12mL) to give a precipitate that was collected by vacuum filtration.
References:
Ashton, Trent D.;Ngo, Anna;Favuzza, Paola;Bullen, Hayley E.;Gancheva, Maria R.;Romeo, Ornella;Parkyn Schneider, Molly;Nguyen, Nghi;Steel, Ryan W.J.;Duffy, Sandra;Lowes, Kym N.;Sabroux, Helene Jousset;Avery, Vicky M.;Boddey, Justin A.;Wilson, Danny W.;Cowman, Alan F.;Gilson, Paul R.;Sleebs, Brad E. [Bioorganic Chemistry,2021,vol. 117,art. no. 105359]
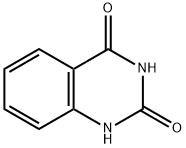
86-96-4
255 suppliers
$22.00/25g

157864-32-9
13 suppliers
inquiry
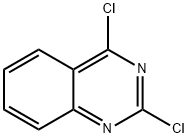
607-68-1
314 suppliers
$10.00/1g

157864-32-9
13 suppliers
inquiry
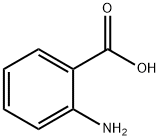
118-92-3
0 suppliers
$23.00/250g

157864-32-9
13 suppliers
inquiry
