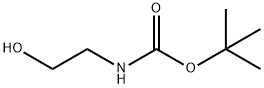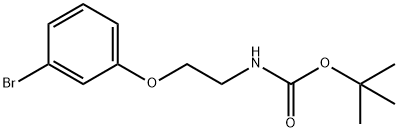
N-Boc-2-(3-bromophenoxy)ethylamine synthesis
- Product Name:N-Boc-2-(3-bromophenoxy)ethylamine
- CAS Number:1098107-26-6
- Molecular formula:C13H18BrNO3
- Molecular Weight:316.19
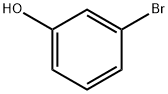
591-20-8
338 suppliers
$10.00/1g
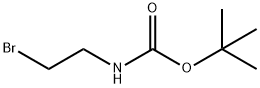
39684-80-5
238 suppliers
$17.30/250mg

1098107-26-6
8 suppliers
inquiry
Yield:1098107-26-6 92%
Reaction Conditions:
with potassium carbonate;potassium iodide in N,N-dimethyl-formamide at 60; for 16 h;Inert atmosphere;
Steps:
tert-butyl (2-(3-bromophenoxy)ethyl)carbamate
To a mixture of 3-bromophenol (1.0 g, 5.78 mmol), potassium carbTo a mixture of 3-bromophenol (1.0 g, 5.78 mmol), potassium carbonate (2.4 g, 17.37 mmol) and potassium iodide (1.0 g, 6.02 mmol) in anhydrous DMF (8 mL) was added tert-butyl (2- bromoethyl)carbamate (2.6 g, 11 .60 mmol) and the reaction mixture stirred under nitrogen for 16 h at 60 °C. The reaction was filtered through celite, the filtrate diluted with EtOAc (20 mL) and washed sequentially with NaOH (10 mL, 0.5 M) and water (2x1 0 mL). The organic layer was dried through aonate (2.4 g, 17.37 mmol) and potassium iodide (1.0 g, 6.02 mmol) in anhydrous DMF (8 mL) was added tert-butyl (2- bromoethyl)carbamate (2.6 g, 11 .60 mmol) and the reaction mixture stirred under nitrogen for 16 h at 60 °C. The reaction was filtered through celite, the filtrate diluted with EtOAc (20 mL) and washed sequentially with NaOH (10 mL, 0.5 M) and water (2x1 0 mL). The organic layer was dried through hydrophobic frit and the filtrate concentrated under reduced pressure. The resulting oil was loaded in DCM (5 mL) and purified on a silica cartridge (50 g) using a gradient of 0-50 % EtOAc in cyclohexane over 10 CV. The appropriate fractions were combined and the solvent removed by rotary evaporation to give the title compound as a colourless oil (1 .69 g, 5.34 mmol, 92%).LCMS (2 mm Formic): Rt= 1.20 mi [MH]= 316/318.
References:
WO2014/140076,2014,A1 Location in patent:Page/Page column 169; 170

591-20-8
338 suppliers
$10.00/1g
![TERT-BUTYL N-[2-(TOSYLOXY)ETHYL]CARBAMATE](/CAS/GIF/158690-56-3.gif)
158690-56-3
61 suppliers
$26.00/1g

1098107-26-6
8 suppliers
inquiry