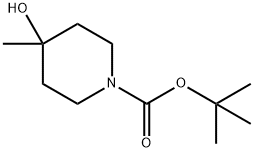
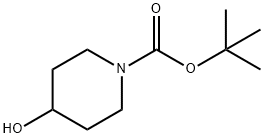
N-BOC-4-METHYL-4-HYDROXY PIPERIDINE synthesis
- Product Name:N-BOC-4-METHYL-4-HYDROXY PIPERIDINE
- CAS Number:406235-30-1
- Molecular formula:C11H21NO3
- Molecular Weight:215.29
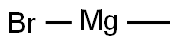
75-16-1
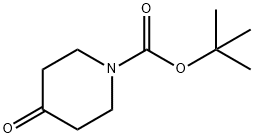
79099-07-3

406235-30-1
a) N-Boc-4-piperidone (1.0 g, 5.0 mmol) was dissolved in anhydrous ether (10 mL) and cooled to -10°C under nitrogen protection. An ether solution of methylmagnesium bromide (3M, 2.5 mL, 7.5 mmol) was slowly added dropwise, and the reaction mixture was stirred for 5 minutes after the drop was completed. The cooling bath was removed and the reaction mixture was allowed to warm to room temperature and stirring was continued for 2 hours. Upon completion of the reaction, the reaction was quenched with saturated aqueous ammonium chloride solution (10 mL) and subsequently diluted with water (20 mL) and ether (20 mL). The aqueous phase was separated and extracted with ether (2 x 30 mL), and the combined organic phases were washed with brine (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to afford N-BOC-4-methyl-4-hydroxypiperidine as a colorless oil (1.05 g, 97% yield).1H NMR (400 MHz, CDCl3) δ 3.76-3.64 (m, 2H), 3.29 -3.16 (m, 2H), 1.66 (s, 1H), 1.45 (s, 9H), 1.25 (s, 3H), solvent peaks were not clearly attributed.
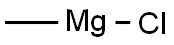
676-58-4
292 suppliers
$15.00/25ml

79099-07-3
0 suppliers
$5.00/5g

406235-30-1
115 suppliers
$22.00/250mg
Yield:406235-30-1 99%
Reaction Conditions:
in tetrahydrofuran at -78 - 0; for 2 h;
Steps:
70.A Step A:
tert-butyl 4-hydroxy-4-methylpiperidine-1-carboxylate
To a solution of tert-butyl 4-oxopiperidine-1-carboxylate (1.00 g, 5.02 mmol) in dry THF (25 mL) was added methylmagnesium chloride (2.17 mL, 6.52 mmol) at -78° C.
The reaction mixture was slowly warmed to 0° C. with stirring for 2 hours and quenched with saturated aq. NH4Cl.
The mixture was extracted with EtOAc twice, and the combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo to afford tert-butyl 4-hydroxy-4-methylpiperidine-1-carboxylate (1.08 g, >99%) as a colorless oil, which was used for the next reaction without further purification. 1H-NMR (CDCl3, Varian, 400 MHz): δ 1.27 (3H, s), 1.46 (9H, s), 1.50-1.58 (4H, m), 3.20-3.27 (2H, m), 3.64-3.76 (2H, m).
References:
US2016/168156,2016,A1 Location in patent:Paragraph 0602; 0603
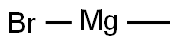
75-16-1
274 suppliers
$12.00/10ml

79099-07-3
0 suppliers
$5.00/5g

406235-30-1
115 suppliers
$22.00/250mg

79099-07-3
0 suppliers
$5.00/5g

406235-30-1
115 suppliers
$22.00/250mg
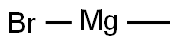
75-16-1
274 suppliers
$12.00/10ml
109384-19-2
420 suppliers
$5.00/1g

406235-30-1
115 suppliers
$22.00/250mg
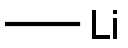
917-54-4
184 suppliers
$52.10/25ml

79099-07-3
0 suppliers
$5.00/5g

406235-30-1
115 suppliers
$22.00/250mg