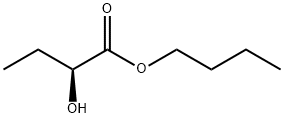
(S)-Butyl 2-hydroxybutanoate synthesis
- Product Name:(S)-Butyl 2-hydroxybutanoate
- CAS Number:132513-51-0
- Molecular formula:C8H16O3
- Molecular Weight:160.21
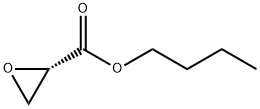
118623-63-5
4 suppliers
inquiry

75-16-1
277 suppliers
$12.00/10ml

132513-51-0
87 suppliers
$497.72/5MG
Yield:132513-51-0 79%
Reaction Conditions:
copper(l) chloride in diethyl ether at -78; for 0.25 h;Product distribution / selectivity;
Steps:
5
Examples 2 to 6 and Comparative Examples 1 to 3 Reaction was performed in a manner similar to Example 1 and under the conditions shown in Table 1. Isolation yields are also given in Table 1. Notably, in all cases, n-butyl (S)-(-)-2,3-epoxypropionate was used in an amount of 200 mg and anhydrous diethyl ether was used as a reaction solvent. [Table 1] Entry Cu catalyst eq. Reaction conditions yield Ex. 2 CuI 0.075 -78°C, 3.5 h 184 mg (83%) Ex. 3 CuI 0.15 -20°C, 15 min 143 mg (64%) Ex. 4 CuI 0.15 -78°C, 15 min 220 mg (99%) Ex. 5 CuCl 0.15 -78°C, 15 min 177 mg (79%) Ex. 6 CuBr 0.15 -78°C, 15 min 169 mg (76%) Comp. Ex. 1 CuI 0 -78°C, 2.5 h - Comp. Ex. 2 CuI 0.75 -78°C, 15 min 92 mg (41%) Comp. Ex. 3 CuI 1.5 -78°C, 2.5 h - -: Difficulty in product isolation and purification due to complicated product system. As is clear from Table 1, the highest yield was attained under the conditions: -78°C, 15 minutes, and use of catalyst (copper (I) iodide) in an amount of 0.15 mol equivalent. When an excessive amount of catalyst was employed, yield decreased, and a complicated product system was formed. When no catalyst was used, a complicated product system including an unreacted substance was formed; i.e., no target product was obtained.
References:
EP1908747,2008,A1 Location in patent:Page/Page column 6; 7

1055325-40-0
0 suppliers
inquiry

132513-51-0
87 suppliers
$497.72/5MG

118623-63-5
4 suppliers
inquiry

132513-51-0
87 suppliers
$497.72/5MG
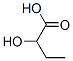
565-70-8
8 suppliers
inquiry

71-36-3
1091 suppliers
$14.00/25mL

132513-51-0
87 suppliers
$497.72/5MG
![Butanoic acid, 2-hydroxy-3-[(phenylmethyl)thio]-, butyl ester, (2R,3S)-](/CAS/20210305/GIF/1447829-03-9.gif)
1447829-03-9
0 suppliers
inquiry

132513-51-0
87 suppliers
$497.72/5MG