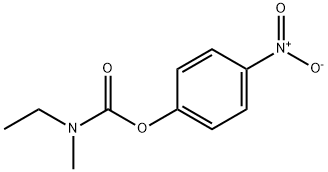
N-Ethyl-N-methyl-O-(4-nitrophenyl)carbamate synthesis
- Product Name:N-Ethyl-N-methyl-O-(4-nitrophenyl)carbamate
- CAS Number:90870-20-5
- Molecular formula:C10H12N2O4
- Molecular Weight:224.21
Yield:90870-20-5 76%
Reaction Conditions:
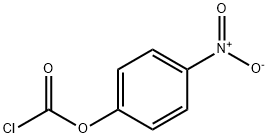
with triethylamine in N,N-dimethyl-formamide at 20;Inert atmosphere;
Steps:
10
Triethylamine (12.84 g, 127.11 mmol) and p-nitrophenyl chloroformate (63.56 mmol, 12.8 g) were added to a room temperature solution of N-ethylmethylamine (2.50 g, 42.37 mmol) in dry DMF (70 mL) under an argon atmosphere. After 2 h, the reaction mixture was quenched with water, diluted with EtOAc (200 mL), washed with water (2 × 100 mL), and brine (75 mL). All volatiles were removed under reduced pressure and the residue was purified by SiO2 column chromatography using 10% EtOAc/hexanes to afford compound 4-nitrophenyl ethyl(methyl)carbamate (5.8 g, 76%) as a yellow oil. TLC: 20% EtOAc/hexanes, Rf ≈ 0.50; 1H NMR (CDCl3, 300 MHz) δ 8.18-8.21 (m, 2H), 7.25-7.29 (m, 2H), 3.37-3.46 (m, 2H), 3.05 and 2.97 (s, 3H for two rotamers in 60/40 ratio), 1.17-1.22 (m, 3H).
References:
EP2208720,2010,A1 Location in patent:Page/Page column 34