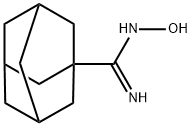
N-HYDROXY-ADAMANTANE-1-CARBOXAMIDINE synthesis
- Product Name:N-HYDROXY-ADAMANTANE-1-CARBOXAMIDINE
- CAS Number:53658-91-6
- Molecular formula:C11H18N2O
- Molecular Weight:194.27
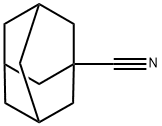
23074-42-2
137 suppliers
$21.21/1gm:

53658-91-6
17 suppliers
inquiry
Yield:53658-91-6 72%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydrogencarbonate in methanol; for 4 h;Reflux;
Steps:
1
46[0100] N-hydroxyadamantanecarboximidamide (46)A suspension of 1-nitrile adamantane (0.96g, 6mmol), hydroxylamine hydrochloride (0.5g, 7.2mmol) and NaHCO3 (0.6Og, 7.2mmol) in CH30H (15ml) was refluxed for 4hrs, and then concentrated in vacuo to remove CH3OH. The residue was extracted with ethyl acetate, washed with brine and dried over MgSO4 to afford crude mixture, which was subsequently purified by flash chromatography (5%-15% CH3OH/CH2C12) to give 46 as white solid (0.84g, Yield: 72%). 1H-NMR (360 MHz, CD3OD) δ 2.05 (br s, 4H), 2.01 (br s, 3H), 1.86-1.84 (m, 2H), 1.80-1.74 (m, 6H); 13C-NMR (90 MHz, CD3OD) δ 41.09, 40.87, 37.92, 36.88, 29.88, 28.78; ESI- MS: Calculated for C11H18N2O (M + H)+ 195.3, Found: 195.7. HCI
References:
WO2011/22191,2011,A1 Location in patent:Page/Page column 31