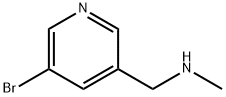
N-Methyl-(5-bromopyrid-3-yl)methylamine synthesis
- Product Name:N-Methyl-(5-bromopyrid-3-yl)methylamine
- CAS Number:73335-64-5
- Molecular formula:C7H9BrN2
- Molecular Weight:201.06
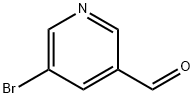
113118-81-3
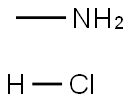
593-51-1

73335-64-5
GENERAL STEPS: 1.8 g (27 mmol) of methylamine hydrochloride and 0.4 g (6 mmol) of sodium cyanoborohydride were added sequentially to a 20 mL methanol solution containing 1 g (5.4 mmol) of 5-bromopyridine-3-carbaldehyde. The reaction mixture was stirred at room temperature for 48 hours. After completion of the reaction, the precipitate was removed by filtration and the filtrate was concentrated to dryness under reduced pressure. The residue was dissolved in dichloromethane and washed with deionized water. After separating the organic phase, the organic phase was washed with dilute aqueous hydrochloric acid. The acid-washed aqueous phase was adjusted to pH 9 with aqueous sodium hydroxide and subsequently extracted with dichloromethane. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with dichloromethane/methanol (9.5:0.5, v/v) as eluent. 0.5 g (50% yield) of N-methyl-(5-bromopyridin-3-yl)methylamine was finally obtained.

113118-81-3
285 suppliers
$5.00/250mg

593-51-1
543 suppliers
$21.00/100g

73335-64-5
50 suppliers
$25.00/100mg
Yield:73335-64-5 92.5%
Reaction Conditions:
with sodium cyanoborohydride;triethylamine in methanol at 0 - 20; for 1.5 h;
Steps:
1 Step 1
To stirred mixture of 5-bromonicotinaldehyde (LVII) (2.0 g, 10.75 mmol), methanamine hydrochloride (0.06 mL, 32.26 mmol) and TEA (6.74 mL, 48.38 mmol) in MeOH (20 mL) was added NaCNBH3 (2.03g, 32.26mmol) at 0°C and the mixture was stirred for 30 min. to 1 h at room temperature. Reaction mixture was quenched with minimum amount of aqueous saturated ammonium chloride solution, concentrated under vacuum and the residue was adsorbed on silica gel, purified by chromatography (010% 7N NH3-MeOH/Chloroform) to obtain 1-(5- bromopyridin-3-yl)-N-methylmethanamine (LVIII) (2.0 g, 9.95 mmol, 92.5% yield) as an yellow syrup. ESIMS found for C7H9BrN2 m/z 201.0 (Br79M+H).
References:
WO2019/241540,2019,A1 Location in patent:Paragraph 0367-0368