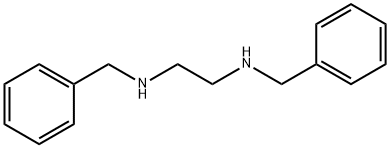
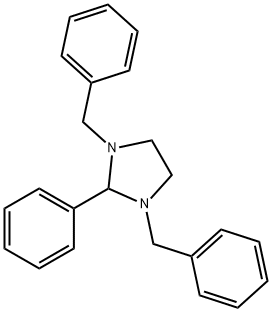
N,N'-Bis(phenylmethyl)-1,2-ethanediamine synthesis
- Product Name:N,N'-Bis(phenylmethyl)-1,2-ethanediamine
- CAS Number:140-28-3
- Molecular formula:C16H20N2
- Molecular Weight:240.34
Yield:140-28-3 70%
Reaction Conditions:
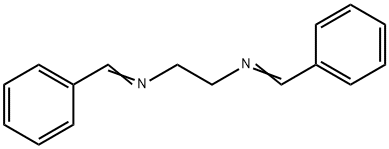
Stage #1:benzaldehyde;ethylenediamine with sodium sulfate;triethylamine in ethyl acetate at 20; for 14 h;
Stage #2: with sodium tetrahydroborate in methanol;ethyl acetate for 6 h;
Steps:
N,N'-dibenzyl-1,2-diaminoethane (21) Quantities Used: 13.4 ml (200 mmol) 1,2-diaminoethane 13.9 ml (100 mmol) triethylamine 28.4 g (200 mmol) sodium sulphate 44.5 ml (440 mmol) benzaldehyde 30.3 g (800 mmol) sodium borohydride General Synthesis Instructions: Add a solution of 440 mmol benzaldehyde in 50 ml ethyl acetate in drops to a solution of 200 mmol of the respective α,ω-diaminoalkane, 100 mmol triethylamine and 200 mmol sodium sulphate in 100 ml ethyl acetate under refrigeration and then stir for 14 hours at room temperature. Add 150 ml methanol, then add 800 mmol sodium borohydride in portions under refrigeration. The substance should be added over a period of 6 hours to avoid formation of foam. Siphon off the solid (also use a 3 cm-high silica gel layer as a filtration aid) and rewash four times with 100 ml chloroform each time. Extract the filtrate twice against 200 ml of a 1 N NaOH (solution) and concentrate the organic phase to a small volume. Purify the residue via column chromatography on 200 g silica gel. Elute the apolar impurities with cyclohexane/ethyl acetate (2:1+1 vol % triethylamine). Then elute the product with ethyl acetate/methanol (1:1+1 vol % triethylamine).
References:
Massing, Ulrich;Fichert, Thomas US2003/229037, 2003, A1 Location in patent:Page/Page column 42, 15
104-71-2
48 suppliers
$18.00/250mg

140-28-3
197 suppliers
$10.00/5g

100-52-7
972 suppliers
$17.30/2g
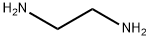
107-15-3
0 suppliers
$11.19/25ML
4597-81-3
1 suppliers
inquiry

140-28-3
197 suppliers
$10.00/5g
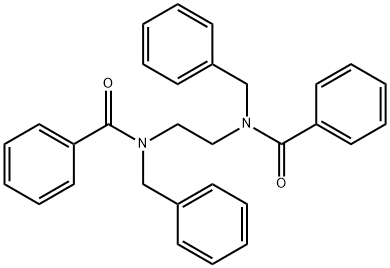
82126-37-2
0 suppliers
inquiry

140-28-3
197 suppliers
$10.00/5g

100-52-7
972 suppliers
$17.30/2g

140-28-3
197 suppliers
$10.00/5g