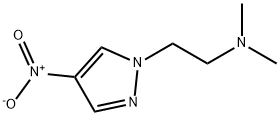
N,N-dimethyl-2-(4-nitro-1H-pyrazol-1-yl)ethanamine synthesis
- Product Name:N,N-dimethyl-2-(4-nitro-1H-pyrazol-1-yl)ethanamine
- CAS Number:1257078-45-7
- Molecular formula:C7H12N4O2
- Molecular Weight:184.2
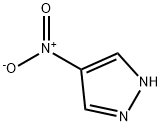
2075-46-9
331 suppliers
$6.00/5g
108-01-0
18 suppliers
$10.00/20mg

1257078-45-7
13 suppliers
inquiry
Yield:1257078-45-7 75%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 0 - 20; for 16 h;Inert atmosphere;
Steps:
i. PREPARATION OF S-77
A cooled solution of S-17 (1.60 g, 14.14 mmol), S-76 (1.0 g, 11.31 mmol) and triphenylphosphine (4.60 g, 17.67 mmol) in THF (50 mL) was charged with DIAD (3.7 mL, 17.67 mmol) dropwise at 0 °C under nitrogen atmosphere. The reaction mixture was stirred for 5 min, cooled to room temperature and then stirred for 16 h. The reaction mixture was partitioned between CH2Cl2(50 mL) and HCl (1 M, 20 mL). The organic phase was separated and was washed with HCl (1 M, 2 × 20 mL). The combined aqueous phase was basified by the addition of 40% NaOH solution and was extracted with CH2Cl2(2 × 50 mL). The combined organic phase was washed with brine (2 × 20 mL). The organic layer was dried over anhydrous Na2SO4and was concentrated under reduced pressure. The crude compound was purified by column chromatography on silica gel using 0- 3% MeOH in CH2Cl2as eluent to afford S-77 (1.20 g, 75%, AMRI lot IN-CKB-G-81) as an off-white solid. The compound was characterized by1H NMR analysis.1H NMR (300 MHz, DMSO- d6): δ 8.87 (s, 1H), 8.25 (s, 1H), 4.26 (t, J = 6.30 Hz, 2H), 2.67 (t, J = 6.30 Hz, 2H), 2.16 (s, 6H).
References:
WO2017/106771,2017,A1 Location in patent:Paragraph 00429

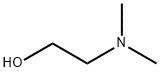
2075-46-9
331 suppliers
$6.00/5g
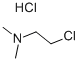
4584-46-7
0 suppliers
$21.69/100g

1257078-45-7
13 suppliers
inquiry

2075-46-9
331 suppliers
$6.00/5g
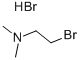
2862-39-7
142 suppliers
$10.00/1g

1257078-45-7
13 suppliers
inquiry