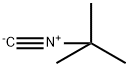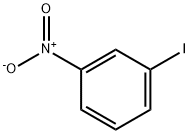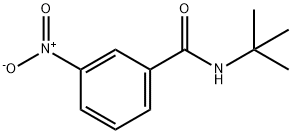
N-(tert-butyl)-3-nitrobenzamide synthesis
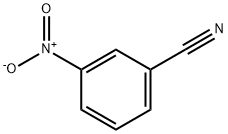
- Product Name:N-(tert-butyl)-3-nitrobenzamide
- CAS Number:10222-93-2
- Molecular formula:C11H14N2O3
- Molecular Weight:222.24
Yield:10222-93-2 89%
Reaction Conditions:
with toluene-4-sulfonic acid at 80; for 5.83333 h;Green chemistry;Ritter Amidation;Reagent/catalyst;Temperature;
Steps:
General Procedure for the Preparation of t-Butylamides in the Presence of p-TSA
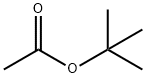
General procedure: A mixture of nitrile (1 mmol), t-butyl acetate (0.116 g, 1 mmol), and p-toluenesulfonic acid (0.2 mmol) was heated in a 10 mL round-bottomed flask equipped with a reflux condenser, at 80°C with stirring. The reactions were complete within 150-360 min as monitored by TLC (eluent, 1:4 EtOAc:n-hexane,). After completion of the reaction, the resulting solid mixture was cooled to room temperature and cooled water (20 mL) was added. The solid products were collected and recrystallized from aqueous ethanol to give the pure products in 69-94% yields based on the starting nitrile. The physical and spectroscopic data of new compounds are as follows.
References:
Nasr-Esfahani, Masoud;Montazerozohori, Morteza;Karami, Zeinab [Organic Preparations and Procedures International,2016,vol. 48,# 4,p. 321 - 327]