
N2,N2-dimethylpyridine-2,4-diamine synthesis
- Product Name:N2,N2-dimethylpyridine-2,4-diamine
- CAS Number:90008-36-9
- Molecular formula:C7H11N3
- Molecular Weight:137.18

36100-42-2
0 suppliers
inquiry

90008-36-9
12 suppliers
inquiry
Yield:90008-36-9 74%
Reaction Conditions:
with hydrogen in methanol at 50; for 22 h;
Steps:
/V2 /V2-dimethylpyridine-2,4-diamine (i51 ):
/V2 /V2-dimethylpyridine-2,4-diamine (i51 ): To a stirred solution of 2-(dimethylamino)-4-nitropyridine 1-oxide (i50) (2 g, 10.92 mmol) in methanol (100 mL), Raney nickel (2 g) was added and the reaction was heated at 50°C under hydrogen pressure for 22h. The progress of the reaction was monitored by TLC. After completion, reaction was filtered through celite and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel (100:200 mesh) column chromatography using 6% methanolic ammonia in dichloromethane as eluent to afford Λ/2 /V2-dimethylpyridine- 2,4-diamine (i51 ) (1.1 g, Yield 74%). 1H NMR (400 MHz, DMSO-d6) δ 2.89 (s, 6H), 5.57 (s, 2H), 5.68 (d, J = 1.8 Hz, 1 H), 5.84 (dd, J = 5.6, 1.8 Hz, 1 H), 7.58 (d, J = 5.6 Hz, 1 H). MS (ESI) m/e (M+1 )+: 138.05
References:
WO2016/124508,2016,A1 Location in patent:Page/Page column 66
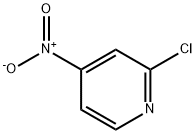
23056-36-2
373 suppliers
$6.00/1g

90008-36-9
12 suppliers
inquiry