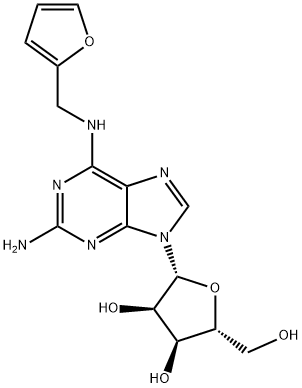
N6-Furfuryl-2-aMinoadenosine synthesis
- Product Name:N6-Furfuryl-2-aMinoadenosine
- CAS Number:26783-39-1
- Molecular formula:C15H18N6O5
- Molecular Weight:362.34
Yield:26783-39-1 96%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine at 125; for 48 h;
Steps:
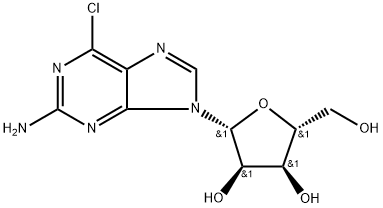
8 4.8 2-Amino-N6-furfuryladenosine (6)
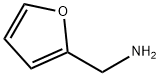
To a solution of 15 (241mg, 0.8mmol) in 2-methoxyethanol (3ml) was added furfurylamine (699mg, 7.2mmol) and DIPEA (931mg, 7.2mmol) and the whole was heated at 125°C for 48h, then evaporated. The residue was chromatographed on silica gel column with CH2Cl2-MeOH (95:5→92:8) and rechromatographed with EtOAc-MeOH (9:1) to give 6 as white solid (278mg, 96% yield). It was next crystallized from EtOAc. 1H, 13C and 15N NMR data of 6 are given in Table1, Table2 and in Supplementary data section. HRMS [M+H]+ calcd for C15H19N6O5: 363.1417; found 363.1401.
References:
Baranowski, Daniel;Framski, Grzegorz;Wyszko, Eliza;Ostrowski, Tomasz [Journal of Molecular Structure,2019,vol. 1195,p. 110 - 118]