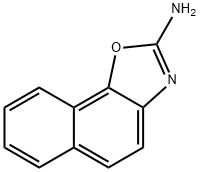
Naphth[2,1-d]oxazol-2-amine synthesis
- Product Name:Naphth[2,1-d]oxazol-2-amine
- CAS Number:858432-37-8
- Molecular formula:C11H8N2O
- Molecular Weight:184.19
Yield:858432-37-8 73%
Reaction Conditions:
with boron trifluoride diethyl etherate in 1,4-dioxane at 80;Inert atmosphere;
Steps:
2.1. General procedure for the preparation of 2-aminobenzoxazoles 2a-g (A)
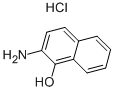
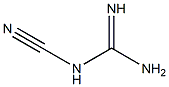
General procedure: To a solution of the corresponding 2-aminophenol (1equiv) and cyanoguanidine (3 equiv) in 1,4-dioxane (30 mL/g of 2-aminophenol)was added boron trifluoride diethyl etherate (3.0 equiv). The reaction was heated under argon atmosphere at 80°C until completion (TLC monitoring, 5-16h). Then, the cooled reaction mixture was quenched with coldsaturated aqueous sodium hydrogen carbonate (100mL/g of 2-aminophenol) and extracted with ethyl acetate (3×100 mL). The combinedorganic layers were dried with anhydrous MgSO4 and evaporated.Depending on purity of the crude material, the residue was washed EtOAc,acetone or another organic solvent, or purified by silicagel flashchromatography (cyclohexane/EtOAc, 100/0 to 0/100, v/v) to afford the desiredproduct. All physical data of the knowncompounds were in agreement with those reported in the literature.
References:
Grytsai, Oleksandr;Druzhenko, Tetiana;Demange, Luc;Ronco, Cyril;Benhida, Rachid [Tetrahedron Letters,2018,vol. 59,# 17,p. 1642 - 1645] Location in patent:supporting information
607-24-9
168 suppliers
$9.00/1g
![Naphth[2,1-d]oxazol-2-amine](/CAS/20210111/GIF/858432-37-8.gif)
858432-37-8
3 suppliers
inquiry
606-41-7
19 suppliers
inquiry
![Naphth[2,1-d]oxazol-2-amine](/CAS/20210111/GIF/858432-37-8.gif)
858432-37-8
3 suppliers
inquiry