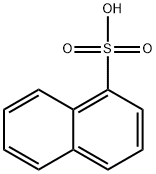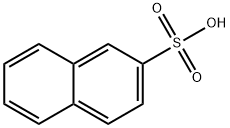
Naphthalene-2-sulfonic acid synthesis
- Product Name:Naphthalene-2-sulfonic acid
- CAS Number:120-18-3
- Molecular formula:C10H8O3S
- Molecular Weight:208.23
Step: With naphthalene as raw material, 98% sulfuric acid is used as sulfonating agent, and it is obtained by sulfonation at 160-166 ℃. During the sulfonation process, a small amount of α-naphthalenesulfonic acid by-product is generated. By heating, the unstable α-naphthalenesulfonic acid can be hydrolyzed at 140-150 °C to remove the sulfonic acid group, and become naphthalene and sulfuric acid. Naphthalene can be blown out. During the hydrolysis of naphthalene, a small amount of lye should be added to neutralize part of the sulfuric acid. It also acts with naphthalenesulfonic acid to form β-naphthalenesulfonic acid sodium salt crystals, which can be filtered to obtain the finished product of Naphthalene-2-sulfonic acid.
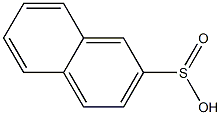
613-49-0
3 suppliers
inquiry

120-18-3
287 suppliers
$7.00/25g
Yield:120-18-3 93%
Reaction Conditions:
with N,N,N,N,N,N-hexamethylphosphoric triamide;oxygen;potassium hydroxide in N,N-dimethyl-formamide at 60; under 760.051 Torr; for 28 h;Sealed tube;Reagent/catalyst;Temperature;Solvent;Pressure;
Steps:
6-2
A reaction vessel was purged with nitrogen, N, N- dimethyl naphthalene-2-sulfonamide (23.5mg, 0.10mmol) and the addition of THF (4mL),It was cooled to 0 ,Red-Al (65wt% toluene solution, 0.30mL, 1.0mmol) was added.It was heated for 22 hours the same mixture at 40 .After completion of the reaction was confirmed by TLC, it was cooled to 0 ,Stirring vigorously DoAdding an appropriate amount of a saturated aqueous solution of sodium sulfate while,Usually with ethyl acetate (10mL × 2)It was carried out of the separation process.The extracted organic layer was washed with saturated sodium chloride water (5mL),After drying with magnesium sulfate, filtered, and concentrated.The resulting concentrate,It continued as it is after the operation without purification.To the resulting concentrate hexamethylphosphoric triamide (HMPA) (1mL),Potassium hydroxide (33.7 mg, 0.60 mmol) was added,Oxygen was the inclusion of about 5 atmospheres (0.5MPa).The mixture was heated at 80 10 hours.After completion of the reaction, the mixture was cooled to room temperature,As it is silica gel column chromatography (chloroform: methanol =1: 1) was separated the product in,71% of 2-naphthalene sulfonic acid potassium salt of purpose yieldObtained in (17.8mg).Further by performing the ion exchange in the amber light,2-naphthalenesulfonic acid of interest was obtained in a high yield.
References:
JP2015/20961,2015,A Location in patent:Paragraph 0029; 0031; 0034
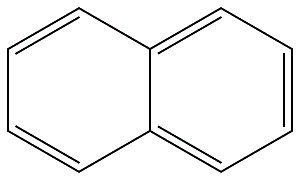
91-20-3
530 suppliers
$14.00/25g

120-18-3
287 suppliers
$7.00/25g
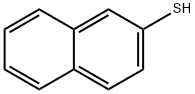
91-60-1
235 suppliers
$15.00/1g

120-18-3
287 suppliers
$7.00/25g
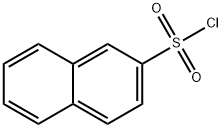
93-11-8
243 suppliers
$14.00/5g

120-18-3
287 suppliers
$7.00/25g