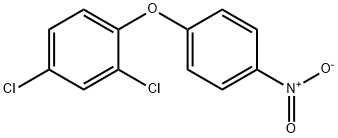
NITROFEN synthesis
- Product Name:NITROFEN
- CAS Number:1836-75-5
- Molecular formula:C12H7Cl2NO3
- Molecular Weight:284.09
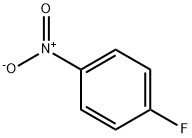
350-46-9
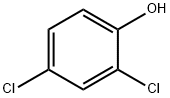
120-83-2

1836-75-5
GENERAL METHOD: A dispersion of 0.69 g (17.3 mmol) of 60% NaH in mineral oil was washed with hexane and subsequently suspended in 20 mL of DMF. A solution of 2,4-dichlorophenol (12.9 mmol) in DMF (20 mL) was added slowly and dropwise. After stirring for 1 hour at room temperature, a DMF (4 mL) solution of p-fluoronitrobenzene (1.5 g, 10.3 mmol) was added. Stirring was continued at room temperature for 1 h. The reaction mixture was then warmed up to 50 °C and maintained for 12 h. The reaction mixture was then heated up to 50 °C. Upon completion of the reaction, the reaction was quenched with saturated NH4Cl solution. The mixture was extracted with ether and the organic layer was washed sequentially with 0.2 M NaOH solution until the aqueous layer was colorless, then with 1 M HCl and saturated brine solution. The organic layer was dried over anhydrous MgSO4, concentrated in vacuum and finally purified by column chromatography using hexane-ethyl acetate (5:1) as eluent to afford the target product 2',4'-dichloro-4-nitro-diphenyl ether.

350-46-9
520 suppliers
$10.60/1gm:

120-83-2
404 suppliers
$12.90/100g

1836-75-5
151 suppliers
$28.00/100mg
Yield:1836-75-5 68%
Reaction Conditions:
Stage #1: 2,4-dichlorophenolwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 20; for 1 h;
Stage #2: 4-Fluoronitrobenzene in N,N-dimethyl-formamide;mineral oil at 20 - 50; for 13 h;
Steps:
Synthesis of compounds (2a-d)
General procedure: 0.69 g (17.3 mmol) of 60% NaH dispersion in mineral oil was washed with hexane and suspended in 20 ml DMF. The solution of corresponding phenol (12.9 mmol) in DMF (20 ml) was then added dropwise. After stirring for 1h at room temperature the solution of 4-nitrobenzene (1.5g, 10.3 mmol) in DMF (4 ml) was added. After further stirring for 1h at room temperature the mixture was heated for 12h at 50 °C. After reaction was complete the reaction mixture was saturated by the solution of NH4Cl. The mixture was extracted with diethyl ether. The organic layer was washed with 0.2 M solution of NaOH until bleaching of the water layer and then washed by 1 M solution of HCl and brine.The organic layer was dried over anhydrous MgSO4. The resulting solution was concentrated in vacuo and purified using column chromatography with hexane-ethyl acetate (5:1) as the eluent to give compounds 2a-d with the following characteristics:
References:
Antipin, Roman L.;Beshnova, Daria A.;Petrov, Rostislav A.;Shiryaeva, Anna S.;Andreeva, Irina P.;Grigorenko, Vitaly G.;Rubtsova, Maya Yu.;Majouga, Alexander G.;Lamzin, Victor S.;Egorov, Alexey M. [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 7,p. 1588 - 1592] Location in patent:supporting information
100-00-5
56 suppliers
$14.00/25g

120-83-2
404 suppliers
$12.90/100g

1836-75-5
151 suppliers
$28.00/100mg
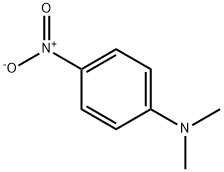
100-23-2
12 suppliers
$36.00/5g

1836-75-5
151 suppliers
$28.00/100mg
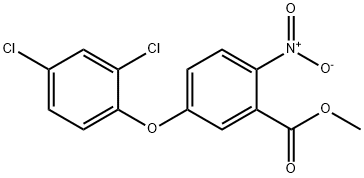
42576-02-3
113 suppliers
$78.59/50mg

1836-75-5
151 suppliers
$28.00/100mg

120-83-2
404 suppliers
$12.90/100g