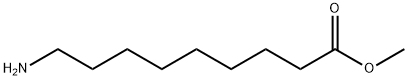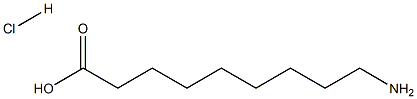
Nonanoic acid,9-amino-, hydrochloride (1:1) synthesis
- Product Name:Nonanoic acid,9-amino-, hydrochloride (1:1)
- CAS Number:23734-86-3
- Molecular formula:C9H20ClNO2
- Molecular Weight:209.7136
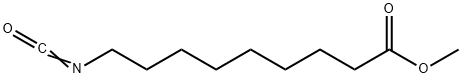
167493-50-7
0 suppliers
inquiry

23734-86-3
1 suppliers
inquiry
Yield:23734-86-3 2 g
Reaction Conditions:
with hydrogenchloride;water for 3 h;Reflux;
Steps:
9-Aminononanoic acid hydrochloride (2b)
(COCl)2 (2.5 ml, 3.67 g, 28.9 mmol) was added to a solution of ester 4 (2.5 g, 11.6 mmol) in CH2Cl2 (20 ml). The reaction mixture was stirred at room temperature for 6 h, then evaporated to dryness. The residue was dissolved in Me2CO (30 ml), and a solution of NaN3 (3.7 g, 56.5 mmol) in H2O (15 ml) was added to the resulting solution with cooling and stirring. The reaction mixture was kept at 0°C for 1 h, then poured into H2O (300 ml) and extracted with AcOEt (3×25 ml). The combined organic layers were dried over MgSO4 and evaporated to dryness. The residue was heated under reflux in PhH (100 ml) for 1 h, then evaporated. Concentrated HCl (15 ml) was added to the resulting residue, the mixture was heated under reflux for 3 h, and evaporated to dryness. The residue was recrystallized from a MeOH-Et2O, 4:1. Yield 2.0 g (84%), white powder, mp 132-133°C (MeOH-Et2O) (mp 133-134°C (MeOH-Et2O)7a). 1H NMR spectrum, δ, ppm: 1.20-1.34 (8H, m, (CH2)4); 1.42-1.60 (4H, m, CH2(CH2)4CH2); 2.14-2.24 (2H, m, CH2CO); 2.68-2.80 (2H, m, CH2N); 7.75-8.05 (3H, m, NH3Cl); 11.99 (1H, s, COOH). Found, %: C 51.61; H 9.69; Cl 16.63; N 6.78. C9H20ClNO2. Calculated, %: C 51.55; H 9.61; Cl 16.91; N 6.68.
References:
Andronova, Valeriya L.;Charushin, Valery N.;Galegov, Georgii А.;Krasnov, Victor P.;Levit, Galina L.;Vozdvizhenskaya, Olga А. [Chemistry of Heterocyclic Compounds,2021,vol. 57,# 4,p. 490 - 497][Khim. Geterotsikl. Soedin.,2021,vol. 57,# 4,p. 490 - 497]
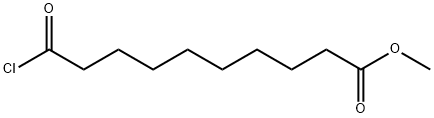
14065-32-8
35 suppliers
$165.00/5g

23734-86-3
1 suppliers
inquiry
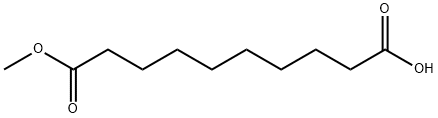
818-88-2
144 suppliers
$14.00/5g

23734-86-3
1 suppliers
inquiry
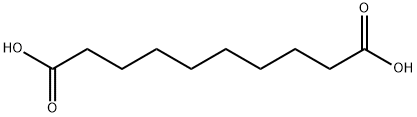
111-20-6
688 suppliers
$5.00/10g

23734-86-3
1 suppliers
inquiry