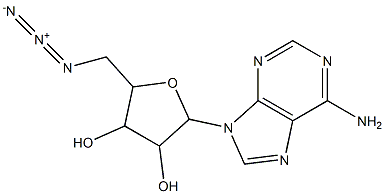
NSC 98778 synthesis
- Product Name:NSC 98778
- CAS Number:737-76-8
- Molecular formula:C10H12N8O3
- Molecular Weight:292.2541
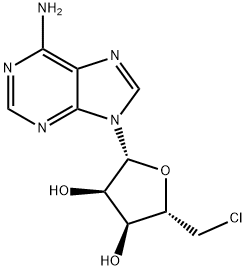
892-48-8
150 suppliers
$6.00/250mg

737-76-8
11 suppliers
inquiry
Yield:737-76-8 393 mg
Reaction Conditions:
with sodium azide in N,N-dimethyl-formamide at 80; for 5 h;Inert atmosphere;
Steps:
Compound 17:
Pyridine (610 L, 7.48 mmol) and thionyl chloride (1.4 mL, 18.7 mmol) were addedat 0 C, over 5 min to a solution of adenosine (1 g, 3.74 mmol) in MeCN (10 mL). The reaction mixturewas stirred at 0 C for 3 h before being warmed to room temperature and stirred for 16 h. The resultingprecipitate was filtered and dissolved in water/MeOH (5:1) and aqueous ammonia (25%, 2 mL) wasadded. The reaction mixture was stirred at room temperature for 30 min and the solvent was removedunder reduced pressure to provide 50-chloroadenosine [66]. The resulting 50-chloroadenosine was thensolubilized in DMF (5 mL) and sodium azide (1.2 g, 18.7 mmol) was added. The reaction mixture washeated at 80 C for 5 h, and cooled to room temperature. The excess of sodium azide was removed byfiltration and the filtrate purified by flash chromatography (DCM/MeOH 9:1) to give 17 as a whitefoam (393 mg, 36% over 2 steps). 1H NMR (500 MHz, CD3OD): 8.29 (s, 1H, H8), 8.21 (s, 1H, H2),6.03 (s, 1H, H10), 4.80-4.78 (m, 1H, H20), 4.40-4.36 (m, 1H, H40), 4.27 (q, J = 5 Hz, 0.5H, H30), 4.18 (q,J = 5 Hz, 0.5H, H30), 3.94 (dd, J = 10, 5 Hz, 0.5H, H50), 3.84 (dd, J = 10, 5 Hz, 0.5H, H50), 3.69-3.62 (m,1H, H50). HRMS (ESI) m/z: calcd for C10H13N8O3 [M + H]+: 293.1110; found: 293.1098. Analyticaldata were in accordance with the literature [67].
References:
Atdjian, Colette;Braud, Emmanuelle;Coelho, Dylan;Ethève-Quelquejeu, Mélanie;Iannazzo, Laura [Molecules,2020,vol. 25,# 14]
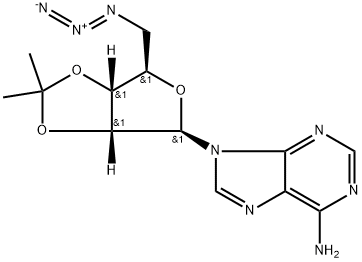
34245-48-2
10 suppliers
inquiry

737-76-8
11 suppliers
inquiry
![9H-Purin-6-amine, 9-[(3aR,4R,6S,6aR)-6-(chloromethyl)tetrahydro-2-oxidofuro[3,4-d]-1,3,2-dioxathiol-4-yl]-](/CAS/20210305/GIF/662131-88-6.gif)
662131-88-6
0 suppliers
inquiry

737-76-8
11 suppliers
inquiry

57610-04-5
0 suppliers
inquiry

737-76-8
11 suppliers
inquiry
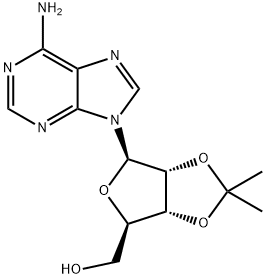
362-75-4
255 suppliers
$6.00/250mg

737-76-8
11 suppliers
inquiry