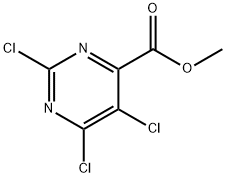
Methyl 2,5,6-trichloropyrimidine-4-carboxylate synthesis
- Product Name:Methyl 2,5,6-trichloropyrimidine-4-carboxylate
- CAS Number:89284-85-5
- Molecular formula:C6H3Cl3N2O2
- Molecular Weight:241.46
91447-90-4
53 suppliers
$352.00/500mg

89284-85-5
64 suppliers
$45.00/10mg
Yield:89284-85-5 83%
Reaction Conditions:
Stage #1: 5-chloro-2,4-dihydroxy-6-methoxycarboxylpyrimidinewith trichlorophosphate at 10;
Stage #2: with N,N-diethylaniline at 0; for 18 h;Reflux;
Steps:
39.1 Process 1) 2,5,6-trichloro-pyrimidine-4-carboxylic acid methyl ester
To 5-chloro-2,4-dihydroxy-6-methoxycarboxylpyrimidine (3 g, 14.67 mmol) was slowly added phosphoryl chloride (POCl3) (55 ml, 0.59 mol) at 10° C.
After cooling to 0° C., N,N'-diethyl aniline (3.5 ml, 22 mmol) was slowly added to the mixture, and the reaction mixture was warmed to room temperature and heated under reflux 18 hours.
After completion of the reaction, the mixture was cooled to room temperature and concentrated under reduced pressure.
After pouring the residue to ice water, the mixture was extracted with ethyl acetate (*3), dried (MgSO4), filtered, and concentrated under reduced pressure.
The residue was purified by silica gel column chromatography (40% EtOAc/hexane) to afford the title compound (2.95 g, 83%).
1H NMR (500 MHz, CDCl3) δ 4.04 (s, 3H)
References:
US2019/159450,2019,A1 Location in patent:Paragraph 0256-0257

91447-90-4
53 suppliers
$352.00/500mg

89284-85-5
64 suppliers
$45.00/10mg