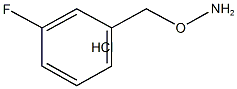
O-(3-Fluoro-benzyl)-hydroxylamine hydrochloride synthesis
- Product Name:O-(3-Fluoro-benzyl)-hydroxylamine hydrochloride
- CAS Number:51572-90-8
- Molecular formula:C7H9ClFNO
- Molecular Weight:177.61
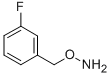
55418-29-6
3 suppliers
inquiry

51572-90-8
48 suppliers
$101.00/500mg
Yield:-
Reaction Conditions:
with hydrogenchloride in diethyl ether;Inert atmosphere;
Steps:
O-(3-fluorobenzyl)hydroxylamine hydrochloride (16)
General procedure: To a solution of alcohol (1mmol) in freshly distilled THF (5ml) was added triphenylphosphine (1.1mmol) and N-hydroxylphthalimide (1.1mmol). After the solution was cooled to 0°C diisopropylazodicarboxylate (1.1mmol) was added dropwise. The solution was allowed to warm to room temperature over 3h. Reaction progress was monitored by TLC (1:1 heptanes:ethyl acetate). Hydrazine monohydrate (1.1mmol) was then added and the solution was allowed to stir for 30min. The resulting reaction mixture was filtered to remove the white precipitate. The filtrate was concentrated and subjected to flash chromatography (1:1 heptanes/ethyl acetate). The resulting product was dissolved in ether and treated with HCl (2.0M solution in ether) to afford the HCl salt of the O-alkylhydroxylamine. Contaminating diisopropyl hydrazinodicarboxylate could be washed away from the HCl salt with dichloromethane. Synthesized from 3-fluorobenzyl alcohol according to the general procedure to afford 16 as white crystals in 81% yield. Mp=245°C (decomp).1H NMR (400MHz, CD3OD) δ 7.45 (t, 1H, ArH, J=8Hz) 7.28-7.15 (m, 3H, ArH), 5.07 (s, 2H, ArCH2). 13C NMR (100MHz, CD3OD) δ 164.22 (d, JC-F=240Hz), 136.91 (d, JC-F=8Hz), 131.80 (d, JC-F=8Hz), 126.04 (d, JC-F=3Hz), 117.31 (d, JC-F=21Hz), 116.85 (d, JC-F=22Hz), 77.12 (d, JC-F=2Hz). GC tR=3.981min.
References:
Malachowski, William P.;Winters, Maria;DuHadaway, James B.;Lewis-Ballester, Ariel;Badir, Shorouk;Wai, Jenny;Rahman, Maisha;Sheikh, Eesha;LaLonde, Judith M.;Yeh, Syun-Ru;Prendergast, George C.;Muller, Alexander J. [European Journal of Medicinal Chemistry,2016,vol. 108,p. 564 - 576]
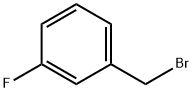
456-41-7
297 suppliers
$13.00/25g

51572-90-8
48 suppliers
$101.00/500mg