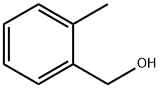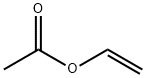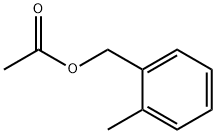
O-METHYLBENZYL ACETATE synthesis
- Product Name:O-METHYLBENZYL ACETATE
- CAS Number:17373-93-2
- Molecular formula:C10H12O2
- Molecular Weight:164.2
Yield: 98%
Reaction Conditions:
with poly(4-vinylpyridine) perchlorate in neat (no solvent) at 20; for 0.366667 h;
Steps:
2.5 Typical procedure of acetylation
General procedure: The substrate (alcohol, phenol or amine; 1.0 mmol) was treated with Ac2O (2.0 mmol) in the presence of P(4-VPH)ClO4 (50 mg) at room temperature under solvent-free conditions and magnetic stirring. After completion of the reaction as indicated by TLC, the mixture was diluted with Et2O (25 ml) and the catalyst allowed to settle down. The supernatant ethereal solution was decanted off, the catalyst washed with Et2O (2 ml) and the combined ethereal solution concentrated under vacuum to afford the product, identical(mp, IR, 1H and 13C NMR, and GC-MS) to an authentic sample of acetylated product. The recovered catalyst was dried at 50 °C under vacuum for 2 h. The recovered catalyst, after drying, was reused for four more consecutive acetylation reactions of benzyl alcohol (1.0 mmol) affording 96, 96, 94, and 94% yields, respectively, in 22, 23, 23, and 25 min (Scheme 2).
References:
Khaligh, Nader Ghaffari [Journal of Molecular Catalysis A: Chemical,2012,vol. 363-364,p. 90 - 100]
95-47-6
616 suppliers
$10.00/25g
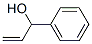
4393-06-0
35 suppliers
inquiry

17373-93-2
25 suppliers
inquiry
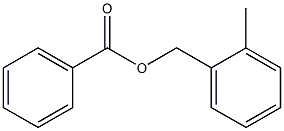
38418-11-0
1 suppliers
inquiry
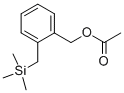
100642-59-9
10 suppliers
inquiry

17373-93-2
25 suppliers
inquiry