8-(Benzyloxy)-8-oxooctanoic acid synthesis
- Product Name: 8-(Benzyloxy)-8-oxooctanoic acid
- CAS Number:15570-39-5
- Molecular formula:C15H20O4
- Molecular Weight:264.32
Yield:15570-39-5 71%
Reaction Conditions:
with dmap;dicyclohexyl-carbodiimide in dichloromethane at 20; for 1 h;Steglich Esterification;
Steps:
8-(Benzyloxy)-8-oxooctanoic acid(1)
To a solution of octanedioic acid (1 g, 5.7 mmol) and benzylic alcohol (0.31 g, 2.9 mmol) in dichloromethane (25 mL) were added 4-dimethylaminopyridine (0.39 g, 3.2 mmol) andN,N'-dicyclohexylcarbodiimide (0.66 g, 3.2 mmol). The mixture was stirred at room temperature for 1 h and concentratedin vacuo. Water (40 mL) was then added and the mixture was extracted with ethyl acetate (3 x 40 mL). The combined organic layers were washed with water (2 x 75 mL) and concentratedin vacuo. The obtained crude product was purifiedviasilica gelcolumn chromatography (cyclohexane/ethyl acetate, 80/20) affording1as a translucent solid (0.545 g, 71% yield).1H NMR (CDCl3, 300 MHz) δ 7.39-7.30 (m, 5H), 5.11 (s, 2H), 2.38-2.31 (m, 4H), 1.70-1.58 (m, 4H), 1.37-1.31 (m, 4H).13C NMR (CDCl3, 75 MHz) δ 178.9 (Cq), 173.7 (Cq), 136.2 (Cq), 128.7 (2 CH), 128.3 (3 CH), 66.3 (CH2), 34.4 (CH2), 33.9 (CH2), 28.8 (CH2), 28.8 (CH2), 24.9 (CH2), 24.6 (CH2). LR-MS (DCI/NH3) m/z calculated for C15H21O4(M+H+)265.15, found 265.2 (M+H+), 282.2 (M+NH4+).
References:
Ouji, Manel;Nguyen, Michel;Mustière, Romain;Jimenez, Tony;Augereau, Jean-Michel;Benoit-Vical, Fran?oise;Deraeve, Céline [Bioorganic and Medicinal Chemistry Letters,2021,vol. 39,art. no. 127884] Location in patent:supporting information
505-48-6
447 suppliers
$8.00/10g

15570-39-5
21 suppliers
inquiry

505-48-6
447 suppliers
$8.00/10g

100-51-6
1432 suppliers
$5.00/100g

42413-23-0
4 suppliers
inquiry
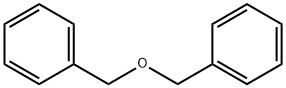
103-50-4
328 suppliers
$5.00/25g

15570-39-5
21 suppliers
inquiry