Olopatadine Impurity 2 synthesis
- Product Name:Olopatadine Impurity 2
- CAS Number:56427-76-0
- Molecular formula:C19H18O4
- Molecular Weight:310.34
55453-87-7
348 suppliers
$5.00/25mg
67-63-0
1609 suppliers
$17.00/25ML

56427-76-0
23 suppliers
inquiry
Yield:56427-76-0 96%
Reaction Conditions:
toluene-4-sulfonic acidReflux;
Steps:
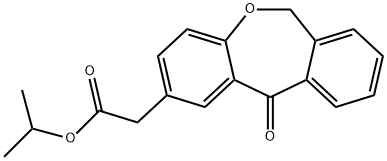
2 6,11-Dihydro-11-oxodibenz[b,e]oxepin-2-isopropyl acetate
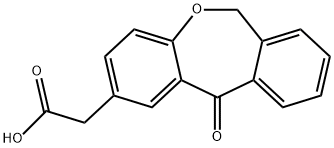
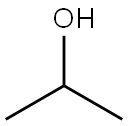
10 g (0.037 moles) of 6,11-dihydro-11-oxodibenz[b,e] oxepin-2-acetic acid were dissolved in 150 ml of isopropanol (iPrOH) and 2 g (0.01 moles) of p-toluenesulfonic acid (p-TsOH) were added to this solution. The resulting solution was heated under reflux, distilling 100 ml of iPrOH from the reaction medium. The reaction was cooled to 40-45°C and 1 ml (0.007 ml) of Et3N was added. Then, the reaction mixture was left to cool to 20-25°C and stirring was maintained at this temperature for 30 minutes. Then, the suspension was left to cool to 5-10°C, it was filtered and the resulting product was washed with iPrOH. 11 g (0.035 moles, 96%) of a white solid identified as the compound of the title were obtained, the spectroscopic properties of which are: 1H-NMR (CDCl3, 400 MHz), δ: 1.21 (d, 6H); 3.59 (s, 2H); 4.12 (m, 1H); 5.11 (s, 2H), 6.97 (d, 1H); 7.29 (d, 1H); 7.38 (m, 2H); 7.47 (m, 1H); 7.84 (d, 1H); 8.08 (d, 1H) ppm. 13C-NMR (CDCl3, 400 MHz), δ: 21.78 (2); 40.22; 68.35; 73.55; 120.95; 125.10; 127.93; 129.18; 129.42; 132.34; 132.72; 135.55; 136.31; 140.39; 160.40; 170.90; 171.37; 190.71 ppm. MS, M++1: 311.12
References:
EP2145882,2010,A1 Location in patent:Page/Page column 13

55453-87-7
348 suppliers
$5.00/25mg

56427-76-0
23 suppliers
inquiry