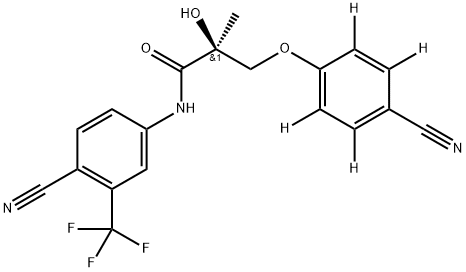
Ostarine(MK-2866) synthesis
- Product Name:Ostarine(MK-2866)
- CAS Number:1202044-20-9
- Molecular formula:C19H14F3N3O3
- Molecular Weight:389.33
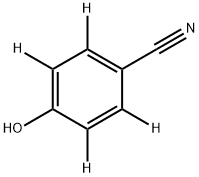
1025089-21-7
12 suppliers
$504.35/5MG
206193-17-1
26 suppliers
inquiry

1202044-20-9
100 suppliers
inquiry
Yield: 59.9%
Reaction Conditions:
with sodium carbonate in acetone for 3 h;Reflux;
Steps:
9
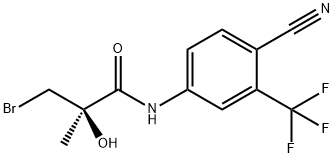
A mixture of bromoamide ((2R)-3- bromo-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide, 50 g, 0.14 mol), anhydrous Na2CO3 (59.04 g, 0.43 mol), and 4-cyanophenol-<£* (25.44 g, 0.21 mol) in 500 mL of acetone was heated to reflux for 3 h and then concentrated under reduced pressure to give a solid. The resulting residue was treated with 500 mL of H2O and then extracted with EtOAc (2 x 300 mL). The combined EtOAc extracts were washed with 10% NaOH (4 X 200 mL) and brine. The organic layer was dried over MgSO4 and then concentrated under reduced pressure to give an oil which was treated with 300 mL of ethanol and an activated carbon. The reaction mixture was heated to reflux for 1 h and then the hot mixture was filtered through Celite. The filtrate was concentrated under reduced pressure to give an oil. This oil was purified by column chromatography using CH2Cl2/Et0Ac (80:20) to give an oil which was crystallized from CH2Cl2/hexane to give 33.2 g (59.9%) of deuterated (S)-_V-(4-cyano-3- (trifluoromethyl)phenyl)-3-(4-cyanophenoxy)-2-hydroxy-2-methylpropanamide as a colorless solid (a cotton type).[00571] 1H NMR (CDC13/TMS) δ 1.63 (s, 3H, CH3), 3.35 (s, 1H,OH), 4.07 (d, J = 9.04 Hz, IH, CH), 4.51 (d, J = 9.04 Hz, IH, CH), 6.97 - 6.99 (m, 2Η, ArH), 7.57-7.60 (m, 2Η, ArH), 7.81 (d, J = 8.55 Hz, IH, ArH), 7.97 (dd, J = 1.95, 8.55 Hz, IH, ArH), 8.12 (d, J = 1.95 Hz, IH, ArH), 9.13 (bs, 1Η, NH). Calculated Mass: 389.10, [M-H]" 388.1. Mp: 92-94 °C.
References:
GTX, INC. WO2009/155481, 2009, A1 Location in patent:Page/Page column 123