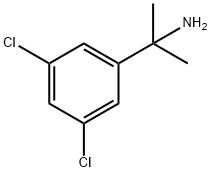
OTAVA-BB 1287452 synthesis
- Product Name:OTAVA-BB 1287452
- CAS Number:129960-45-8
- Molecular formula:C9H11Cl2N
- Molecular Weight:204.1
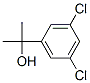
68575-35-9
30 suppliers
$412.00/1g

129960-45-8
48 suppliers
$31.00/250mg
Yield: 70.7%
Reaction Conditions:
with sulfuric acid;acetic acid in ethyl acetate
Steps:
R.2 Preparation of 3,5-dichloro-α,α-dimethylbenzylamine
REFERENCE EXAMPLE 2 Preparation of 3,5-dichloro-α,α-dimethylbenzylamine To 7 ml of acetic acid, 2.83 g (57.8 mmol) of sodium cyanide was gradually added at a temperature not higher than 20° C. To the suspension thereby obtained, 7 ml of an acetic acid solution of 7 ml of concentrated sulfuric acid was dropwise added at not higher than 20° C., and the mixture was stirred at room temperature for one hour. Then, 10.3 g (50 mmol) of 3,5-dichloro-α,α-dimethylbenzyl alcohol was dropwise added thereto at not higher than 20° C. The reaction mixture was stirred at room temperature for two days, and then poured into ice water. The mixture was alkalified with potassium carbonate and extracted with ethyl acetate The ethyl acetate solution was washed with water, dried and then concentrated. The solid thereby obtained was washed with n-hexane and collected by filtration to obtain 8.2 g (yield: 70.7%) of N-(3,5-dichloro-α,α-dimethylbenzyl)formamide as white needle-like crystals. Melting point: 115-117° C.
References:
Kumiai Chemical Industry Co., Ltd.;Ihara Chemical Industry Co., Ltd. US5006157, 1991, A
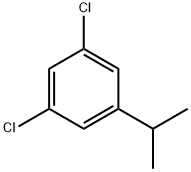
65432-04-4
11 suppliers
inquiry

129960-45-8
48 suppliers
$31.00/250mg