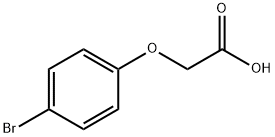
p-Bromophenoxyacetic acid synthesis
- Product Name:p-Bromophenoxyacetic acid
- CAS Number:1878-91-7
- Molecular formula:C8H7BrO3
- Molecular Weight:231.04
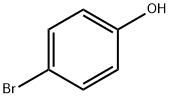
106-41-2
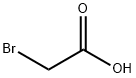
79-11-8

1878-91-7
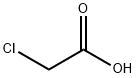
The general procedure for the synthesis of 4-bromophenoxyacetic acid from 4-bromophenol and chloroacetic acid was as follows: a mixture of NaOH (0.04 mol, 1.60 g), deionized water (20 mL), and ethanol (20 mL) was added to a 150 mL three-necked flask, followed by the slow addition of 4-bromophenol (0.04 mol, 6.92 g). After 20 minutes of reaction under stirring, aqueous sodium chloroacetate solution was added dropwise. The reaction mixture was heated to 105 °C and refluxed for 5 hours. After completion of the reaction, it was cooled to room temperature and the pH of the mixture was adjusted to 1-2 with dilute hydrochloric acid. the precipitate was collected by filtration and washed several times with dilute hydrochloric acid. Finally, the product was purified by recrystallization and dried under vacuum to give 4-bromophenoxyacetic acid as a white solid.
Yield:1878-91-7 95%
Reaction Conditions:
with sodium hydroxide in waterReflux;
Steps:
1 1.1. Synthesis of ZCL278 and its Analogs: 4-(3-(2-(4-bromo-phenoxy)-acetyl)-thioureido)-N-(4,6-dimethylpyrimidin-2-yl)-benzenesulfonamide (BA1-12) C21H20BrN5O4S2 (Bromo)
Equimolar (5-7 mmol) NaOH and substituted phenol was dissolved in 15 mL water followed by the addition of equimolar (5-7 mmol) NaOH and bromoacetic acid in 15 mL water. The solution was refluxed for 4-8 h, allowed to cool to RT, acidified with concentrated H2SO4 and extracted with ethyl acetate (3×15 mL). The organic layer was extracted with saturated NaHCO3 (3×20 mL). The aqueous layer was acidified with con. HCl followed by extraction with ethyl acetate (3×15 mL), dried over anhydrous MgSO4 and solvent removed under vacuum resulting in the substituted phenoxyacetic acid (e.g., compound 1d of Scheme 1, above). A solution of the phenoxyacetic acid in 20 mL SOCl2 and a drop of dimethyl formamide was refluxed for 3-6 h then excess SOCl2 removed by distillation to give the crude acyl chloride (e.g., compound 1e of Scheme 1, above). A solution of acyl chloride in 15 mL anhydrous acetone was added to a solution of sodium isothiocyanate in ice cold anhydrous acetone and stirred at RT for 3 h to give the acyl isothiocyanate followed by the addition of 4-amino-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide. After stirring overnight, the solution was poured onto ice and recrystallized in dichloromethane and methanol to give the thiourea product. 4-Bromophenol (1 g, 5.8 mmol) to afford 10 (4-bromophenoxy)acetic acid (1.27, 95%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J=11.1 Hz, 2H), 6.81 (d, J=9.1 Hz, 2H), 4.67 (s, 2H). 10 (4-bromophenoxy)acetic acid (1.5 g, 6.5 mmol) to afford the title thiourea (0.06 g, 2%) as a white 12 solid. 1H NMR (400 MHz, DMSO-d6) δ 12.30 (s, 1H), 11.76 (s, 1H), 8.04 (d, J=8.7 Hz, 2H), 7.90 (d, J=8.7 Hz, 2H), 7.53 (d, J=9.04 Hz, 2H), 7.00 (d, J=9.08 Hz, 2H), 6.81 (s, 1H), 4.95 (s, 2H), 2.31 (1, 6H). 13C NMR (DMSO-d6) δ 178.3, 169.6, 157.0, 132.2, 128.6, 123.5, 116.8, 112.7, 66.1.
References:
East Carolina University;Lu, Qun;Aguilar, Byron;Zhu, Yi;Chen, Yan-Hua US2019/91184, 2019, A1 Location in patent:Paragraph 0272; 0275
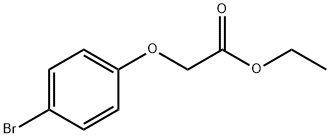
6964-29-0
37 suppliers
$40.00/5 g

1878-91-7
226 suppliers
$9.72/5g

106-41-2
498 suppliers
$13.00/5g
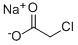
3926-62-3
299 suppliers
$16.69/250g

1878-91-7
226 suppliers
$9.72/5g
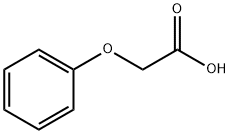
122-59-8
456 suppliers
$5.00/25g

1878-91-7
226 suppliers
$9.72/5g