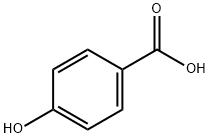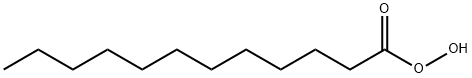
peroxylauric acid synthesis
- Product Name:peroxylauric acid
- CAS Number:2388-12-7
- Molecular formula:C12H24O3
- Molecular Weight:216.3172
Yield:2388-12-7 100%
Reaction Conditions:
with dihydrogen peroxide in water at 20; pH=10 - 11;
Steps:
8 Determining the Peracid Kinetics of Para-Decanoyloxybenzoic Acid (DOBA) from Inventive Example 1 and from Comparative Examples 2 and 3
Example 8 Determining the Peracid Kinetics of Para-Decanoyloxybenzoic Acid (DOBA) from Inventive Example 1 and from Comparative Examples 2 and 3 [0063] The peracid kinetics were determined by means of iodometric titration using sodium thiosulfate solution. [0064] The basis for the measurement is that para-decanoyloxybenzoic acid (DOBA) and hydrogen peroxide react in aqueous solution to form perdecanoic acid and para-hydroxybenzoic acid (if inorganic peroxides are used, they react in aqueous solution to form hydrogen peroxide). The reaction between DOBA and hydrogen peroxide takes place rapidly and quantitatively in dilute aqueous solution at a pH of 10 to 11 and at 20° C. The perdecanoic acid formed can then be determined by iodometry, along with the hydrogen peroxide which is present in excess. The perdecanoic acid is substantially more reactive than hydrogen peroxide and, in a weakly acidic medium and at a low temperature, it undergoes immediate oxidation with added iodide I- (added in the form of potassium iodide, for example) to form iodine I2. The iodine formed can then be titrated with sodium thiosulfate. The corresponding amount of perdecanoic acid can then be calculated from the amount of iodine found. [0065] The specific procedure was as follows: [0066] 1 liter of deionized water at 20° C. was introduced in a 2 liter glass beaker and stirred. 1.5 g of sodium percarbonate and 8 g of a standard laundry detergent (“IEC 60 456 Type A*” from WFK Testgewebe GmbH) were added and subjected to preliminary dissolution for 2 minutes. Then 0.25 g of the DOBA under analysis was added. After 3 minutes, 50 ml were pipetted off and introduced into a 250 ml glass beaker, onto 50 g of ice made from deionized water and 10 ml of acetic acid (20% strength by weight aqueous solution). Then 5 ml of aqueous potassium iodide solution (10% strength by weight aqueous solution) were added, and titration took place with sodium thiosulfate solution (0.01 molar aqueous solution). [0067] Titration was carried out using a Titrino DMS 716 or Basic 794 (metrohm) with a 50-way changeover unit and keyboard, and also a Ti Stand 727 (metrohm) with drawn burette tip, stirring rod, and combined platinum electrode. [0068] The next samples were taken after particular times following the addition of DOBA, and were titrated as described above. As sampling time goes on, the amount of perdecanoic acid approaches a maximum value, and then remains constant in the case of samples taken later. This maximum value for the amount of perdecanoic acid is set at 100%. The amounts of perdecanoic acid for the other samples are then expressed in relation to this 100%.
References:
US2013/211131,2013,A1 Location in patent:Paragraph 0063; 0064; 0065; 0066; 0067; 0068; 0069
143-07-7
784 suppliers
$5.00/25g

2388-12-7
2 suppliers
inquiry
137846-25-4
0 suppliers
inquiry

2388-12-7
2 suppliers
inquiry
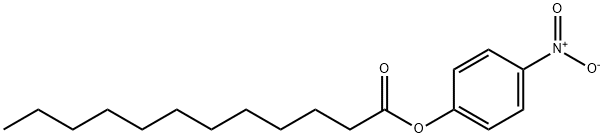
1956-11-2
87 suppliers
$17.00/1g

2388-12-7
2 suppliers
inquiry