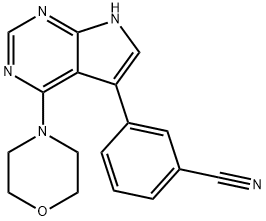
PF-06447475 synthesis
- Product Name:PF-06447475
- CAS Number:1527473-33-1
- Molecular formula:C17H15N5O
- Molecular Weight:305.33

110-91-8
![Benzonitrile, 3-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-](/CAS/20210305/GIF/1527475-89-3.gif)
1527475-89-3

1527473-33-1
1. In a dry reaction flask, morpholine (871 mg, 10 mmol) and N,N-diisopropylethylamine (2.6 g, 20 mmol) were sequentially added to a solution of n-butanol (100 mL) containing 3-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-5-yl)benzonitrile (2.5 g, 9.8 mmol). 2. The reaction mixture was heated to reflux with continuous stirring for 3 hours. 3. Upon completion of the reaction, the solvent was removed by rotary evaporator under reduced pressure. 4. The residue was purified by silica gel column chromatography with the eluent being a solvent mixture of ethyl acetate and petroleum ether (1:1, v/v). 5. After collection of the target fraction, the target fraction was further recrystallized using ethyl acetate and tert-butyl methyl ether to give the white solid product 3-(4-morpholino-7H-pyrrolo[2,3-d]pyrimidin-5-yl)benzonitrile. 6. Yield: 770 mg, 2.52 mmol, 26%. 7. 7. Product characterization: LCMS m/z 306.0 [M + H]+. 1H NMR (400 MHz, DMSO-d6) δ 12.34 (br s, 1H), 8.41 (s, 1H), 7.99-8.02 (m, 1H), 7.89 (br d, J = 8 Hz, 1H), 7.76 (br d, J = 7.5 Hz, 1H), 7.71 (s, 1H), 7.81 (s, 1H), 7.82 (br d, J = 7.5 Hz, 1H). 7.71 (s, 1H), 7.68 (dd, J = 7.8, 7.8 Hz, 1H), 3.44-3.50 (m, 4H), 3.11-3.17 (m, 4H).

110-91-8
711 suppliers
$9.00/1g
![Benzonitrile, 3-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-](/CAS/20210305/GIF/1527475-89-3.gif)
1527475-89-3
0 suppliers
inquiry

1527473-33-1
88 suppliers
$25.00/1mg
Yield:1527473-33-1 26%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in butan-1-ol for 3 h;Reflux;
Steps:
4.4 Step 4. Synthesis of 3-[4-(morpholin-4-yl)-7H-pyrrolo[2, 3-d]pyrimidin-5-yl]benzonitrile(4).
Morpholine (871 mg, 10 mmol) and N,N-diisopropylethylamine (2.6 g, 20 mmol) were added to a solution of 3-(4-ch loro-7H-pyrrolo[2, 3-d]pyrim idin-5-yl)benzonitrile (C14) (2.5 g, 9.8 mmol) in n-butanol (100 mL), and the reaction mixture was heated at reflux for 3 hours. Solvents were removed in vacuo and the residue was purified using chromatography on silica gel (Eluent: 1:1 ethyl acetate petroleum ether). Subsequent recrystallization from ethyl acetate and tert-butyl methyl ether afforded the product as a white solid. Yield: 770 mg, 2.52 mmol, 26%. LCMS m/z 306.0 [M+H]. 1H NMR (400MHz, DMSO-d6) 12.34 (br s, 1H), 8.41 (s, 1H), 7.99-8.02 (m, 1H), 7.89 (br d, J=8 Hz,1 H), 7.76 (br d, J=7.5 Hz, 1 H), 7.71 (s, 1 H), 7.68 (dd, J=7.8, 7.8 Hz, 1 H), 3.44-3.50 (m,4H), 3.11-3.17 (m, 4H).
References:
PFIZER INC.;GALATSIS, Paul;HAYWARD, Matthew Merrill;HENDERSON, Jaclyn;KORMOS, Bethany Lyn;KURUMBAIL, Ravi G;STEPAN, Antonia Friederike;VERHOEST, Patrick Robert;WAGER, Travis T.;ZHANG, Lei WO2014/1973, 2014, A1 Location in patent:Page/Page column 56; 57; 58
![7H-Pyrrolo[2,3-d]pyrimidine, 4-chloro-5-iodo-7-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20210111/GIF/1207543-30-3.gif)
1207543-30-3
6 suppliers
inquiry

1527473-33-1
88 suppliers
$25.00/1mg
![4-Chloro-5-iodo-7H-pyrrol[2,3-d]pyrimidine](/CAS/GIF/123148-78-7.gif)
123148-78-7
271 suppliers
$8.00/1g

1527473-33-1
88 suppliers
$25.00/1mg
150255-96-2
360 suppliers
$8.00/1g

1527473-33-1
88 suppliers
$25.00/1mg