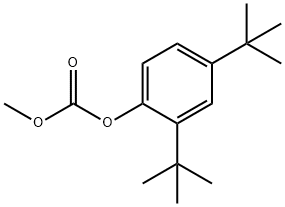
Phenol, 2,4-bis(1,1-diMethylethyl)-5-nitro- synthesis
- Product Name:Phenol, 2,4-bis(1,1-diMethylethyl)-5-nitro-
- CAS Number:873055-57-3
- Molecular formula:C14H21NO3
- Molecular Weight:251.32
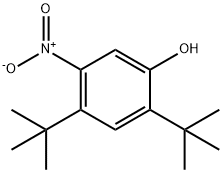
873055-55-1
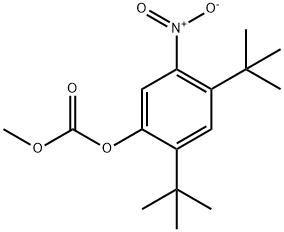
873055-56-2

873055-57-3

20039-94-5
The general procedure for the synthesis of 2,4-di-tert-butyl-5-nitrophenyl methyl carbonate and compound (CAS: 873055-56-2) from 2,4-di-tert-butyl-5-nitrophenol and 2,4-bis(tert-butyl)-6-nitrophenol was as follows. ester mixture (4.2 g, 12.9 mmol) was dissolved in methanol (65 mL) and potassium hydroxide (2.0 g, 36 mmol) was added. The reaction mixture was stirred at room temperature for 2 hours. Subsequently, the reaction mixture was acidified to pH 2-3 by addition of concentrated hydrochloric acid. the acidified mixture was partitioned between water and ether. The ether layer was separated, dried over magnesium sulfate, concentrated, and purified by column chromatography (0-5% ethyl acetate-hexane) to afford 2,4-di-tert-butyl-5-nitrophenol (1.31 g, two-step yield 29%) and 2,4-di-tert-butyl-6-nitrophenol.1H NMR (400 MHz, DMSO-d6) data for 2,4-di-tert-butyl-5-nitrophenol: δ 10.14 (s, 1H, OH), 7.34 (s, 1H), 6.83 (s, 1H), 1.36 (s, 9H), 1.30 (s, 9H).1H NMR (400 MHz, CDCl3) data of 2,4-di-tert-butyl-6-nitrophenol: δ 11.48 (s, 1H), 7.98 (d, J = 2.5Hz, 1H). 7.66 (d, J = 2.4 Hz, 1H), 1.47 (s, 9H), 1.34 (s, 9H).

873055-55-1
91 suppliers
$60.00/50mg

873055-57-3
68 suppliers
inquiry
Yield: 98%
Reaction Conditions:
with sodium hydroxide in methanol;water at 20; for 5 h;
Steps:
1 Preparation of Compound 4 (2,4-di-tert-butyl-5-nitrophenol)
To a stirred solution of compound 3 (3.0 g, 9.7 mmol) in MeOH (20 mL) was added NaOH (776 mg, 19.4 mmol) dissolved in H2O (5 mL), and the reaction mixture was stirred at room temperature for 5 h and acidified with aq.2N HCl to pH 2-3. The resulting precipitate was collected by suction filtration. The desired regioisomer, compound 4 (2.4 g, 98%) was obtained as pale yellow solid and used in the next step after drying under high vacuum. 1H NMR (400 MHz, CDCl3) δ7.42 (s, 1H), 6.69 (s, 1H), 5.20 (s, 1H), 1.41 (s, 9H), 1.38 (s, 9H).
References:
ORPHOMED, INC.;SINGH, Nikhilesh Nihala US2021/9556, 2021, A1 Location in patent:Paragraph 0057; 0060
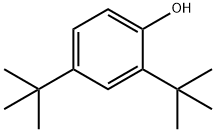
96-76-4
392 suppliers
$6.00/25g

873055-57-3
68 suppliers
inquiry

873055-55-1
91 suppliers
$60.00/50mg

873055-56-2
4 suppliers
inquiry

873055-57-3
68 suppliers
inquiry

20039-94-5
26 suppliers
inquiry