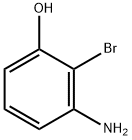
Phenol, 3-aMino-2-broMo- synthesis
- Product Name:Phenol, 3-aMino-2-broMo-
- CAS Number:100367-36-0
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02
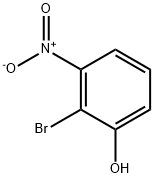
101935-40-4

100367-36-0
General procedure for the synthesis of 3-amino-2-bromophenol from 2-bromo-3-nitrophenol: 2-bromo-3-nitrophenol (3.35 g, 15.4 mmol), acetic acid (35 mL), and powdered iron (4.5 g, 77.2 mmol) were mixed in ethanol (50 mL), and the reaction was stirred overnight under reflux conditions. Upon completion of the reaction, the mixture was diluted with 100 mL of water and neutralized with potassium carbonate. Subsequently, extraction was carried out with dichloromethane (DCM). The organic phase was dried with anhydrous sodium sulfate and concentrated under vacuum to give a brown oily crude product. The crude product was purified by column chromatography by dry loading onto a silica gel column using a 0-50% ethyl acetate/isohexane gradient elution. The target fraction was collected and concentrated in vacuum to give 3-amino-2-bromophenol (1.27 g, 6.75 mmol, 43.9% yield) as a light yellow solid. [00409] 1H NMR (DMSO-d6): δ 7.02 (t, J = 8.0 Hz, 1H), 6.45 (dd, J = 1.6 and 8.0 Hz, 1H), 6.38 (dd, J = 1.6 and 8.0 Hz, 1H), 5.44 (br s, 1H), 4.89 (br s, 2H).
56619-93-3
89 suppliers
$22.00/1g

100367-36-0
54 suppliers
$50.00/100mg
Yield:-
Steps:
Multi-step reaction with 3 steps
1: 1.) n-BuLi, 2.) bromine / 1.) THF, hexane, 0 deg C, 2.5 h, 2.) THF, hexane, -78 deg C, 1 h
2: 1.) AlCl3 / 1.) CS2, reflux, 3 h, 2.) 70 deg C, 2 d
3: 61 percent / ethanolic HCl / 12 h / 80 °C
References:
Kozikowski, Alan P.;Ma, Dawei;Du, Linh;Lewin, Nancy E.;Blumberg, Peter M. [Journal of the American Chemical Society,1995,vol. 117,# 25,p. 6666 - 6672]

101935-40-4
137 suppliers
$9.00/100mg

100367-36-0
54 suppliers
$50.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

100367-36-0
54 suppliers
$50.00/100mg
168335-61-3
2 suppliers
inquiry

100367-36-0
54 suppliers
$50.00/100mg
603-85-0
270 suppliers
$15.00/5g

100367-36-0
54 suppliers
$50.00/100mg