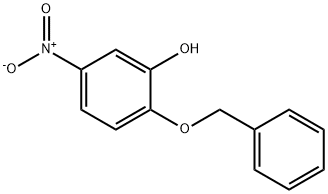
Phenol, 5-nitro-2-(phenylmethoxy)- synthesis
- Product Name:Phenol, 5-nitro-2-(phenylmethoxy)-
- CAS Number:2242-35-5
- Molecular formula:C13H11NO4
- Molecular Weight:245.23
Yield:2242-35-5 80%
Reaction Conditions:
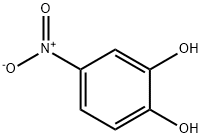
Stage #1: 1,2-dihydroxy-4-nitrobenzenewith sodium hydride in N,N-dimethyl-formamide at -10 - 20; for 1 h;
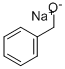
Stage #2: benzyl bromide in N,N-dimethyl-formamide at -10 - 20; for 16 h;
Steps:
2-benzyloxy-5-nitrophenol (1b)
A solution of 4-nitrocatechol (5g, 32.2 mmol) in DMF (25 mL) was added dropwise to a suspension of NaH (774 mg, 32.2 mmol) in DMF (50 mL) cooled at 10 °C. The reaction mixture was stirred for 1 h at room temperature. After cooling at 10 °C, a solution of benzyl bromide (3.8 mL, 32.2 mmol) in DMF (20 mL) was slowly added over a period of 30 min. After 16 h at room temperature, the mixture was diluted with ethyl ether and washed with 1N HCl. The organic phase was concentrated and the resultant residue purified on silica gel (cyclohexane/DCM 8/2) to give 1b (6.32 g, 80%) as a light yellow solid. The analytical data are reported in Table 1 and in the Supplementary material.
References:
Bolchi, Cristiano;Bavo, Francesco;Pallavicini, Marco [Synthetic Communications,2017,vol. 47,# 16,p. 1507 - 1513] Location in patent:supporting information
2620-44-2
158 suppliers
$31.00/5g
20194-18-7
31 suppliers
$131.00/25ml

2242-35-5
2 suppliers
inquiry

5876-96-0
0 suppliers
inquiry

2242-35-5
2 suppliers
inquiry