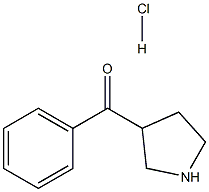
Phenyl-3-pyrrolidinyl-Methanone HCl synthesis
- Product Name:Phenyl-3-pyrrolidinyl-Methanone HCl
- CAS Number:25503-87-1
- Molecular formula:C11H14ClNO
- Molecular Weight:211.688
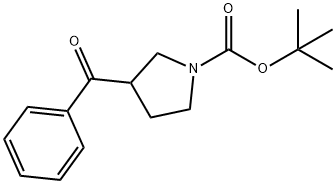
952685-33-5

25503-87-1
General procedure for the synthesis of phenyl(pyrrol-3-yl)methanone hydrochloride from tert-butyl 3-benzoylpyrrolidine-1-carboxylate: Trifluoroacetic acid (10 mL) was added to a solution of tert-butyl 3-benzoylpyrrolidine-1-carboxylate (0.600 g, 2.18 mmol) in dichloromethane (40 mL), and the reaction mixture was stirred for 1 hour at room temperature. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure to give a residue. This residue was dissolved in methanol (10 mL), followed by the addition of a solution of 1 M hydrochloric acid in ether (100 mL). The resulting slurry was stirred at room temperature for 1 h and then concentrated to dryness under reduced pressure to give phenyl(pyrrolidin-3-yl)methanone hydrochloride (0.435 g, 94% yield) as a tan solid. The mass spectrum (APCI positive ion mode) showed m/z 176 (100%) [M+H]+.

952685-33-5
1 suppliers
inquiry

25503-87-1
27 suppliers
$8.00/250mg
Yield:25503-87-1 94%
Reaction Conditions:
Stage #1: 1,1-dimethylethyl 3-benzoyl-1-pyrrolidinecarboxylatewith trifluoroacetic acid in dichloromethane at 18 - 25; for 1 h;
Stage #2: with hydrogenchloride in methanol;diethyl ether at 18 - 25; for 1 h;
Steps:
3
To a solution of tert-butyl 3-benzoylpyrrolidine-l- carboxylate (0.600 g, 2.18 mmol) in DCM (40 inL) was added TFA (10 mL) and the mixture was stirred at room temperature for 1 hour. Concentration yielded a residue which was dissolved in MeOH (10 mL) and IM HCl in Et2O (100 mL) was added. The slurry was stirred for 1 hour at room temperature then concentrated to dryness to give phenyl (pyrrolidin-3-yl)methanone hydrochloride (0.435 g, 94%) as a tan solid: m/z (APCI pos) 176 (100%) [M+H] .
References:
WO2008/11130,2008,A2 Location in patent:Page/Page column 147; 149