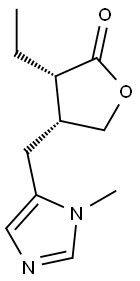
PILOCARPINE synthesis
- Product Name:PILOCARPINE
- CAS Number:92-13-7
- Molecular formula:C11H16N2O2
- Molecular Weight:208.26
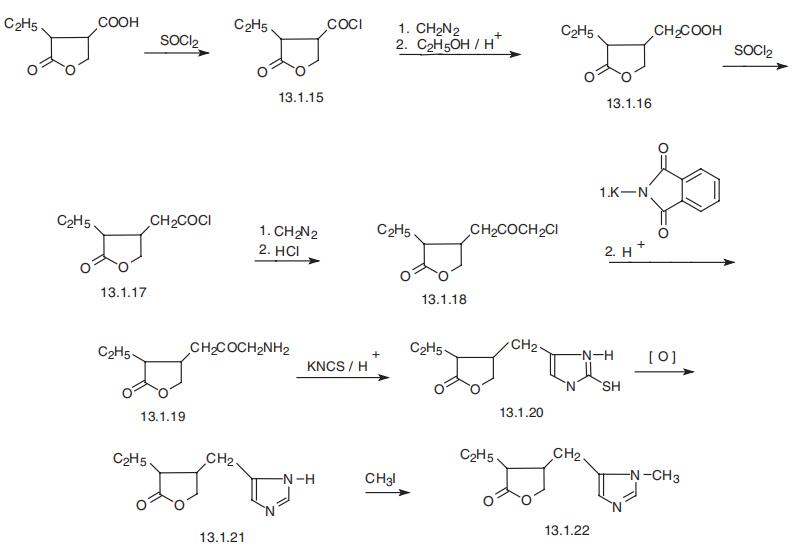
![2(3H)-Furanone, 4-[(2,3-dihydro-3-methyl-2-thioxo-1H-imidazol-4-yl)methyl]-3-ethyldihydro-](/CAS/20210305/GIF/858221-10-0.gif)
858221-10-0
0 suppliers
inquiry

92-13-7
108 suppliers
$220.00/300mg
Yield:92-13-7 69.3 mg (84%)
Reaction Conditions:
with ammonium hydroxide;nitric acid;sodium nitrite in water
Steps:
1 Example 1
Example 1 0.10 g(0.40 mmol) of 3-ethyl-dihydro-4-[(1-methyl-1H-2-mercaptoimidazol-5-yl)methyl]-2(3H)-furanone and 0.3 ml (4.7 mmol) of concentrated nitric acid were added to 2.0 ml of water, and the mixture was stirred for 5 minutes under ice-cooling. To the mixture was added 42 mg(0.60 mmol) of sodium nitrite and the stirring was continued for additional 30 minutes. Then, the temperature of the mixture was raised to room temperature followed by stirring for further 2 hours. After the reaction was complete, the reaction mixture was made alkaline by adding 0.5 ml of 35% aqueous ammonia and extracted with 5 ml of chloroform. The chloroform extract was dried over anhydrous sodium sulfate and evaporated to give 69.3 mg (84%) of pilocarpine as white crystals. The crystals were dissolved in ethanol, filtrated to remove insolubles, 0.1 ml of concentrated hydrochloric acid added, and evaporated to give 77 mg (79%) of white crystals having a m.p. of 200-203°C. 1H-NMR and mass spectrum (molecular ion peak at 208) of the crystals thus obtained are identical with those of the authentic sample pilocarpine hydrochloride.
References:
MITSUI PETROCHEMICAL INDUSTRIES, LTD. EP404175, 1990, A1
120230-62-8
0 suppliers
inquiry

92-13-7
108 suppliers
$220.00/300mg
![Butanedioic acid, 2-bromo-3-ethyl-, 4-methyl ester, [S-(R*,S*)]- (9CI)](/CAS/GIF/146499-97-0.gif)
146499-97-0
2 suppliers
inquiry

92-13-7
108 suppliers
$220.00/300mg
80436-91-5
8 suppliers
inquiry

92-13-7
108 suppliers
$220.00/300mg