
PIPERIDINE, 4-(5-PHENYL-1H-IMIDAZOL-2-YL)- synthesis
- Product Name:PIPERIDINE, 4-(5-PHENYL-1H-IMIDAZOL-2-YL)-
- CAS Number:737766-57-3
- Molecular formula:C14H17N3
- Molecular Weight:227.3
![tert-butyl 4-[(2-oxo-2-phenylethyl)carbamoyl]piperidine-1-carboxylate](/CAS/20180629/GIF/1082949-95-8.gif)
1082949-95-8
1 suppliers
inquiry

737766-57-3
2 suppliers
inquiry
Yield:737766-57-3 64%
Reaction Conditions:
with ethanol;ammonium chloride at 160; under 10501.1 Torr; for 11 h;microwave irradiation;
Steps:
36
Preparation 36; 4-(4-Phenyl- lH-imidazol-2-yl)piperidine hydrochloride; Microwave heat a mixture of tert-butyl 4-(2-oxo-2-phenylethylcarbamoyl)- piperidine-1-carboxylate (1.0 g, 2.88 mmol) and NH4Cl (0.462 g, 8.65 mmol) in ethanol (12 mL) at 300W, 160 0C, and 14 bar for 11 h in a Personal Chemistry microwave. Cool the mixture to room temperature, add silica gel (5 mL), and concentrate the mixture in vacuo. Purify the residue by silica gel chromatography (80 g RediSep column, elute with a gradient of 0% through 100% CMA/methylene chloride over 60 min, 60 mL/min) to provide 4-(4-phenyl-lH-imidazol-2-yl)piperidine (0.418 g, 64%) as an off- white solid. Add 4-(4-phenyl-lH-imidazol-2-yl)piperidine (0.060 g, 0.26 mmol) to methylene chloride (15 mL) and concentrated hydrochloric acid (28 μL). Stir the mixture for 45 min, then concentrate the mixture in vacuo to provide 4-(4-phenyl- lH-imidazol-2- yl)piperidine hydrochloride (0.070 g, 99%). MS (APCI): m/z = 228 [M + H].
References:
WO2008/140947,2008,A1 Location in patent:Page/Page column 22

79099-07-3
0 suppliers
$5.00/5g

737766-57-3
2 suppliers
inquiry
![1-[(Benzyloxy)carbonyl]piperidine-4-carboxylic acid](/CAS/GIF/10314-98-4.gif)
10314-98-4
265 suppliers
$6.00/1g

737766-57-3
2 suppliers
inquiry
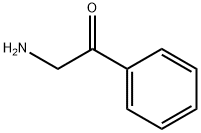
613-89-8
76 suppliers
$338.00/100g

737766-57-3
2 suppliers
inquiry