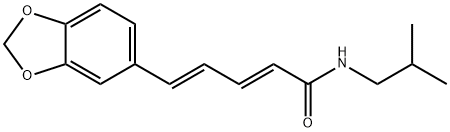
piperlonguminine synthesis
- Product Name:piperlonguminine
- CAS Number:5950-12-9
- Molecular formula:C16H19NO3
- Molecular Weight:273.33
Yield:5950-12-9 86%
Reaction Conditions:
with triethylamine at 20; for 5 h;Cooling with ice;
Steps:
General Procedure for Preparation of Piperic AcidAmides (4-24)
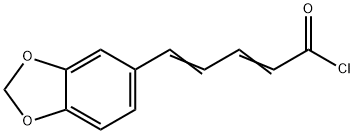
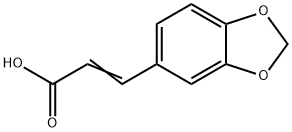
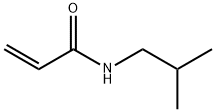
General procedure: Oxalyl chloride (1.27 g, 10 mmol) was addedto a mixture of piperic acid (218 mg, 1.0 mmol) in CH2Cl2(5 mL) and the mixture was stirred at room temperature for3 h. The solvent and excess oxalyl chloride was then evaporatedunder reduced pressure. The crude acid chloride generatedwas dissolved in CH2Cl2 or N,N-dimethylformamide(DMF) (2 mL), and was added dropwise to a mixture of theappropriate amine or its hydrochloride salt (1.2 mmol) andEt3N (808 mg, 8 mmol) in CH2Cl2 or DMF (5 mL) under icecooling.The reaction mixture was stirred for 5 h at roomtemperature. Ice-water was added to the mixture, which wassubsequently extracted with CHCl3. The organic layer wasdried over Na2SO4 and the solvent was evaporated under reducedpressure. The residue was purified by silica gel columnchromatography (CHCl3 : MeOH : aq. NH3=100 : 1 : 0.1) to givethe corresponding piperic acid amides.
References:
Takao, Koichi;Miyashiro, Takaki;Sugita, Yoshiaki [Chemical and Pharmaceutical Bulletin,2015,vol. 63,# 5,p. 326 - 333]
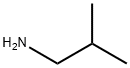
78-81-9
187 suppliers
$19.60/250ml
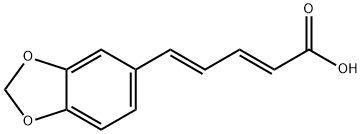
136-72-1
32 suppliers
$65.00/50mg

5950-12-9
76 suppliers
$42.00/5mg
![Acetamide, 2-[[(2E)-3-(1,3-benzodioxol-5-yl)-2-propen-1-yl]sulfonyl]-N-(2-methylpropyl)-](/CAS/20210305/GIF/736947-73-2.gif)
736947-73-2
0 suppliers
inquiry

5950-12-9
76 suppliers
$42.00/5mg

136-72-1
32 suppliers
$65.00/50mg

5950-12-9
76 suppliers
$42.00/5mg